Introduction and Table of Contents
Here’s my week-by-week guide to Unit 1 of AP Bio. I’m following this scope and sequence. Please feel free to email me for ideas and support, or to set up a time to talk one-to-one.
- Week 1: Course opening, Key Themes, Natural Selection
- Week 2: Properties of Water, Standard Error
- Week 3: Biochemistry Part 1 (Monomers and Polymers, Carbohydrates, Lipids)
- Week 4: Proteins and Nucleic Acids
Week 1: Course Opening, Key Themes of Biology, Understanding Natural Selection
Your first week of AP Bio! Beginnings are important, so this week, I’ll walk you through each day.
During the first week, you should try to accomplish a few things.
- Connect with your students. You’re about to take your students on a hero’s journey. They’re going to face obstacles, overcome all types of difficulties, and come out of this with a radically transformed version of life. You’re the guide. In this first week, you want to set the tone and get your students inspired.
- Teach the Big Picture (the College Board’s Four Big Ideas) This provides a cognitive scaffold that reduces fragmentation and which makes the learning more meaningful.
- Teach natural selection. Natural selection is the most important idea in biology. Start teaching it now. Our introductory tutorial, key themes of biology, sets the stage for this, and you can consolidate this understanding with several activities discussed below.
- Set up/communicate your basic procedures and policies. That includes getting your students registered on Learn-Biology.com.
Objectives for Week 1
After week 1, my course closely follows the College Board’s objectives as listed in their course and exam description. But during week 1, I like to lay out the big picture: key course themes, including evolution; understanding structure and function and levels of biological organization; and introducing the scientific method and experimental design.
Here’s a list of what I want my students to be able to do by the end of week 1.
- List and describe the four key themes of AP Biology (evolution; information flow; energy and matter flow; systems and systems interactions).
- Explain how natural selection works to bring about adaptations.
- List and describe the key traits of living things.
- Explain the relationship between structure and function.
- Describe the levels of biological organization.
- List the basic features of experimental design.
Day 1: Connect, Introduce Yourself, and Introduce Course Themes/Big Ideas
Beginnings are important. During this first week, my goals are to
- Connect with my students.
- Inspire them for the journey ahead.
- Show them that I’ll be a competent guide on this journey by offering a well-structured, efficient, and fun first lesson.
I organize the flow of my lesson using slideshows. Here’s a link to the one I’m using for the first week of this year. Note that for about as long as I’ve been teaching, I’ve started my course by teaching my students a class cheer that goes “B-I-O-L-O-G-Y: That’s our classes Battle Cry!” I use that to psyche my students up before a lab, a test, or whenever a psyche-up is needed.
Then I talk for just a little bit about myself. I keep this to about 5 minutes. I’m just trying for a bit of personalization. One thing I share is how, about 15 years ago, I was lucky enough to be able to live in Argentina with my family. While in Argentina, I noticed how the constellation Orion is upside down compared to what we see in North America. Why do I tell this story? Because, in addition to allowing me to share a little bit about myself (I’m a dad, I speak Spanish, etc.), I share how this experience (seeing Orion upside down) changed my perspective, making me think differently about my existence on this planet. I’m hoping, similarly, that the experience my students have in AP Biology is going to shift their perspective and help them think about themselves in a deeper, bigger, more meaningful way. What story do you have?
Next, I put on my motivational speaker hat. AP Bio is a hard course. Our students are facing a big challenge. I’m going to try to inspire them throughout this journey, and Day 1 is the time to start. I present AP Bio as a hero’s journey in which the students are the heroes, and I’m their guide. Note that while I’ll mention the pandemic and SARS-CoV-2 (and I’ll bring it in a lot throughout the year) I’m going to save teaching about the virus for later. I just don’t want to do it during the first few days (because the pandemic has been traumatic for many students, and I want to build more trust before teaching about it). At the same time, based on my experience last year, students are intensely interested in the biology behind the pandemic, so I want to assure students that I will be teaching about it, without going into the details.
I end with an activity called AP Bio sentence matching. You can see my instructions for it in my first week Google slideshow (link is above). Here’s a link to the sentences that I use.
If we still have time after that, I’ll talk to my students about how the course works. This is all embedded in this document which includes weekly agendas and a syllabus (with procedures) from my last year in the classroom, 2022-23.
Day 2: Class Procedures, More Personalization, Introduce Natural Selection
I’ll start class by having my students skim through my weekly agenda (see link immediately above)
Then, I’ll briefly introduce the key ideas of biology. These are the four big ideas that you’ll find in the College Board’s Course and Exam Description. While these ideas (first introduced in 2012) got demoted a bit when the course was redesigned in 2019, they still provide a great framework for understanding biology.
After that, I get my students onto Learn-Biology.com for their first assignment: AP Bio Themes/Key Ideas. Learn-Biology.com is how I teach most of the content in my course, and I need to make sure that my students can log on and complete learning modules. For instructions about how to set up your classes and get your students learning on Learn-Biology.com, go here. This module lays down a conceptual framework for all the biology that’s going to come. It’s also very easy material, and I wanted to pair easy content with a new technology (Learn-Biology.com) for my students.
Day 3: HHMI, Making of the Fittest
On Day 3, I introduce natural selection. Why now? Because I agree with Dobzhansky: it’s the idea that makes everything else in biology make sense.
I’ll do this introduction through HHMI’s Rock Pocket Mouse Activity. The activity consists of a 10-minute video, followed by (or integrated with) a few options for activities.
- This one involves students analyzing a series of cards that they need to put in the correct sequence (which is the one I’ve done in the past).
- This one looks like it has more numerical analysis, and focuses more on the MC1R gene.
The video plus the activities will take up the rest of class, and might even stretch into tomorrow.
Day 4: Finish Rock Pocket Mouse, Start the Peppered Moth
My schedule at BHS has advantages and disadvantages. The advantage is that I get a lot of time. I meet with my students 7 periods a week. That’s once/day, Monday – Friday, and two supplemental periods. The reason we get so much time relates to our disadvantage: my students have never taken a high school biology course (because we’re a “physics first” school, with 9th-grade physics leading to 10th-grade chemistry leading to 11th-grade biology).
I’m telling you this because if you find yourself wondering how I have so much time for activities, lab, etc., it’s because of those extra periods.
On Day 4, I’ll be
- Finishing the Rock Pocket mouse (if needed).
- Checking for understanding of the Key Themes of Biology.
- Doing some brief lecturing about natural selection (see my slideshow).
If your students are ready for another activity about natural selection, turn to the peppered moth. This handout will guide your students through two computer-based activities related to peppered moth evolution. The first is on the Ask a Biologist website from Arizona State University. The second is a NetLogo simulation of peppered moth evolution. Both activities require a class set of Chromebooks and a pretty good Internet connection. If you’re going to do the NetLogo simulation, I strongly suggest that you spend some time playing around with it first.
For an added treat, have your students watch my music video The Ballad of the Peppered Moth. It was originally conceived as a sea shanty, so be prepared to sing.
If you have some time at the end of class, you can play “Grill the teacher. Give your students a few minutes to ask you any question they want about you or the course. If a student goes overboard, you can always say “that’s a bit too personal.” Next question.
Day 5: Setting up your students’ AP Biology Learning Journal
I’m going to start with some checking for understanding of artificial and natural selection. Note that we’ll cover natural selection again when we study evolution in unit 7. For now, my goal is for students to know enough about natural selection so that when we study enzymes, they can explain things like why the shape of an enzyme complements its substrate. Or, when we learn about SARS-CoV-2, to explain why the spike protein of SARS-CoV-2 complements the shape of the ACE receptor, etc., why new variants of SARS-CoV-2 are arising and spreading through various populations.
Use this link to find out about how the AP Biology Learning Journal works. Today is the day when I’m going to dive in, introduce the journal, and have my students get started. By the end of this lesson, my students will have:
- Set up their journals.
- Written their first weekly reflection
- Found and pasted in their first showcase
If there’s time, I’ll also have students comment on one another’s work. You don’t want students to feel time pressure about writing their first reflection, so for this first week, I might just have students comment on each other’s showcase entries.
Week 2: Properties of Water; Understanding Standard Error
Introduction
Last week, we operated on a very big-picture level. This week we’ll start diving into the content of Unit 1. Also, please note that while last week I provided a day-by-day guide, this week (and for the rest of the course) I’ll be suggesting materials for the entire week.
A Note about Basic Chemistry
At my high school, we follow a physics-first sequence. All of my students took a 9th-grade physics course, followed by a 10th-grade chemistry course (at the regular high school or AP level). So my students have all the prerequisite chemistry they need to succeed in AP Biology. If that’s not true of your students, then consider having your students complete my tutorials about Basic Chemistry for Biology Students. These tutorials cover all the chemistry your students need (atoms, molecules, chemical bonding, structural formulas, etc.)
Note that these basic chemistry tutorials are not, by default, assigned to your students, so you’ll have to do this through the “Manage Quizzes/Decks” page on Learn-Biology.com/admin. For instructions about how to assign tutorials, go here.
Objectives for Properties of Water
Here are the key learning objectives for Topic 1.1, Structure of Water and Hydrogen Bonding, in a student-friendly format.
- Describe water’s molecular structure.
- Explain the key physical and chemical properties that result from water’s molecular structure.
- Describe cohesion, adhesion, and surface tension, and explain how these key properties of water result from hydrogen bonding.
- List examples of ways in which living things depend on water’s physical and chemical properties
Related tutorials on Learn-Biology.com
You can access the learning modules related to this topic on Learn-Biology.com at Structure of water and hydrogen bonding. This module has two tutorials: each one includes a video. The first tutorial explains the underlying chemistry. The second is a virtual lab.
Labs and Other Activities
Here’s a link to an actual (non-virtual) Properties of Water introductory lab (which is a qualitative comparison between water and isopropyl alcohol). The lab includes some reading and diagraming which might be redundant if your students are doing it after they do the online tutorial and virtual lab. The advantage of doing the lab first is letting students do some discovery learning, which can be motivating and fun.
The lab, the tutorial, and whatever checking-for-understanding you do might take two full class periods.
Once those concepts are secure, you can turn to teaching about standard error, which is an important statistical concept in the AP Bio curriculum. Ultimately, students just need to understand how to determine when the difference between two sets of data in an experiment is statistically significant. In most tests of this at the AP level, the answer is “when the error bars don’t overlap.”
This table (which is in the lab handout) says it all:
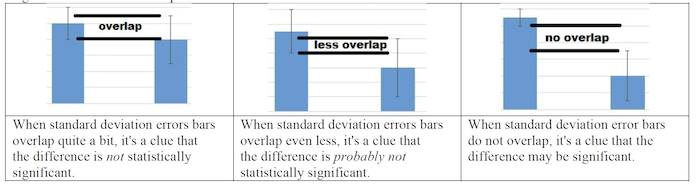
To do this, I take my students through the process of actually calculating standard error. In later labs, we’ll use a Google sheet to combine our data, look at class means, and graph the data with error bars (and let the spreadsheet do all the calculations).
This handout will introduce your students to standard error and error bars. It consists of explanatory readings, followed by practice problems. It culminates in a quantitive version of the water/isopropyl alcohol lab. I modified this from a handout that was posted several years ago in the AP Bio Facebook teacher’s group by Crystal Jenkins Stawiery.
Teaching about standard error takes some time because calculating standard error of the mean is a slow process. I follow this with a second activity about standard error: a case study from the National Center for Case Study Teaching in Science. I have a slightly modified version of this handout (I dropped the last section). The original one is here. If you want the answer key for the tutorial you have to subscribe to the NCCSTS, which I highly recommend. All you need for this activity is a copy of the handout and a ruler.
The first section of this handout is a simulated lab activity where students measure the zone of inhibition generated by growing a variety of bacterial strains (one of which is MRSA) in the presence of one of four antibiotics. The second part talks about how antibiotics work, which is a good preview of topics that will be coming up in biochemistry and cell structure on function. In addition, antibiotic resistance is a great example of natural selection at work. Be sure to help your students to make that connection. If you want to, you can use this section about antibiotic resistance in bacteria from Learn-Biology.com, which comes from our Evidence for Evolution tutorial.
Don’t forget to plant some broccoli
 Sometime during the 2nd week of school, I have my students plant broccoli seeds. Just get some broccoli seeds, and plant them in a small or medium-sized peat pot.
Sometime during the 2nd week of school, I have my students plant broccoli seeds. Just get some broccoli seeds, and plant them in a small or medium-sized peat pot.
This has a couple of purposes.
- It sets up a transpiration lab that I’ll do as soon as the plants get to be big enough (about 4 inches tall). This is one of the main ways that I teach water potential. I also use this as an opportunity for students to look at stomata, which is a great example of feedback regulation and homeostasis.
- Many of my students (even in Berkeley, CA) have never grown a plant from seed. Observing this process (and the unfolding of form that happens during development), provides a great reference point when teaching about development later in the course.
If you plant your seedlings now, they’ll be ready in about 8 weeks (especially if you can grow them under grow lights).
Week 3: Topics 1.2 – 1.6: Biochemistry (Monomers and Polymers, Carbohydrates, Lipids)
Learning Objectives
You can see the College Board’s original objectives for this week’s material in their Course and Exam Description, or my condensed version of the same document. Here they are in student-friendly form:
- Describe the key roles of carbon, nitrogen, and phosphorus in the molecules found in living things.
- Carbon is the key structural atom in all biomolecules
- Nitrogen is in proteins and nucleic acids; phosphorus is in ATP, nucleic acids, and phospholipids.
- Compare and contrast dehydration synthesis and hydrolysis reactions.
- Dehydration synthesis reactions are endergonic and are used to build the complex molecules in living things.
- Hydrolysis reactions are exergonic and are used to release energy and digest polymers into monomers.
- Describe the structure and function of carbohydrates.
- Simple sugars (monosaccharides) are the monomers of carbohydrates. These monomers are combined to create more complex carbohydrates.
- Glucose (a six-carbon simple sugar) is used to power the synthesis of ATP during cellular respiration.
- Describe the structure and function of lipids
- Differences in saturation determine the differences between fats and oils.
- The structure of phospholipids gives them polar/hydrophilic regions and nonpolar/hydrophobic regions.
Biochemistry Tutorials on Learn-Biology.com
Instruction about biochemistry on Learn-Biology.com starts with these two tutorials:
Note that the College Board won’t explicitly test your students about which functional group is which. However, they’re easy to learn, and familiarity with them comes in handy throughout many topics. For example, it’s impossible to understand the principles of protein structure without knowing that some functional groups are polar, others are nonpolar, and others are acidic or basic. My experience is that it’s much easier to teach about proteins if you can say things like: you see this carbonyl group? Remember how it’s polar, with a partial negative charge? When it comes into close contact with this hydroxyl, over here, the two will form a hydrogen bond. When you have dozens of these it can lead this shape to emerge…
It’s worth it to lay down a foundation now. Functional groups will also come in handy when you’re talking about DNA, membranes, phosphorylation cascades, etc.
After carbon and functional groups, move on to these tutorials:
- Topic 1.3: Monomers and Polymers
- Topic 1.4 – 1.6 Overview: The Four Biomolecule Families
- Topic 1.4, Part 1: Carbohydrates
- Topic 1.4, Part 2: Lipids
These tutorials have a lot of video support built in. That includes videos about Carbon, Functional Groups, Monomers and Polymers, Carbohydrates, and an overview video called “The Four Biomolecule Families.”
My tutorials about carbohydrates and lipids go into a lot of detail. This reflects my interest in nutrition and my desire for my students to understand the foods that they eat.
Additional Activities
- Molecular model building lab. This works best if you have a Molymod kit (or any other brand). But if you’re on a budget, you can probably make it work with marshmallows and toothpicks.
- Starch Amylase Lab. This is one of those labs that makes it great to be a biology teacher. Students spit in a test tube filled with starch solution. Salivary amylase hydrolyzes the starch into glucose, which you can test with a test strip, or with Benedict’s reagent. If you can’t stand saliva (or if your school regulations would prohibit it), this lab is not for you. It’s also great for teaching about experimental design and control groups.
- The Biochemistry Basics POGIL You can buy the POGIL packets from Flinn Scientific.
In addition to the POGIL, here’s a link to a worksheet with lots of great diagrams that you can use for in-class checking for understanding. Also, to give carbohydrates a bit of evolutionary context, I like to do this activity about Lactase Persistence/Lactose Intolerance from HHMI: watch this video and complete this worksheet
Week 4: Proteins and Nucleic Acids
Learning Objectives
You can see the College Board’s original objectives for this week’s material in their Course and Exam Description, or in my condensed version of the same document. Look for Topics 1.4 and 1.5. Here they are in a student-friendly form:
- Describe the role and structure of amino acids.
- Amino acids are the monomers of proteins
- They have a central carbon, a hydrogen atom, an amine group, a carboxyl group, and a variable R-group/side chain
- List the types of R groups/side-chains
- hydrophobic, hydrophilic, basic, or acidic.
- Explain how proteins are directional,
- They have an amino terminus and a carboxyl terminus.
- Explain how the four levels of protein structure give rise to a protein’s 3D shape and function
- primary: the sequence of amino acids.
- secondary: interaction between carbonyl and amino residues leading to alpha-helices and beta-pleated sheets.
- tertiary: interactions between R-Groups
- Quaternary: interactions between polypeptide chains.
- Compare and contrast the structure of DNA and RNA.
- Both are polymers of nucleotides, which have 5-carbon sugar, a phosphate group, and a nitrogenous base.
- Both encode information in their sequence of nucleotides.
- In DNA, the sugar is deoxyribose, and the bases are A, T, C, and G.
- In RNA the sugar is ribose, and the bases are A, U, C, and G.
- DNA is a double helix in which the complementary strands are antiparallel; RNA is single-stranded.
- Explain the directionality of DNA, and connect directionality to DNA replication
- DNA (and RNA) has a 5′ to 3′ orientation. During replication, new nucleotides can only be added to the 3′ end of a growing strand.
Tutorials on Learn-Biology.com
This is conceptually difficult material for students, requiring a lot of molecular imagination. But I’ve provided a lot of resources on Learn-Biology.com to support your teaching. This includes
- Topic 1.5: Proteins. This includes an embedded video.
- Topic 1.6. Nucleic Acids
Remember that this is only an overview of nucleic acids. You’ll teach about DNA and RNA in greater depth when you get to AP Bio unit 6 (DNA and molecular genetics).
Additional Activities/Resources
1. Use Flinn’s excellent POGIL on proteins to reinforce this material.
2. To engage your students in some real-world research consider getting your students to play “Foldit,” an educational game that gets users to solve puzzles related to protein folding. The game is computationally intensive, and has to be downloaded onto a computer (there’s no online version). At the very least, offer it as an enrichment option for your students. If they play, they can write about it in their biology learning journals (which I’ll write more about in a future email).
3. HHMI has a biointeractive about the BCR-ABL protein, which results from a translocation mutation that’s associated with chronic myeloid leukemia. While this puts content from much later in the course in front of your students, that’s a good thing: it’ll give you a context for material that you’ll cover in Unit 6.
Unit 1 Cumulative Review
- Student-friendly learning objectives, flashcards, multiple-choice questions, and click-on challenges for Unit 1 are found on this page.
- For additional Unit 1 review, I send my students to AP Classroom to do the Unit 1 progress checks. I also give my students this review worksheet.
