Introduction
Six elements make up most of the mass of living things. You can remember them by the acronym CHNOPS, which stands for Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, and Sulfur.
In what follows, we’ll discuss carbon in detail, because it’s the central element in living things. After that, we’ll take a brief look at the other elements listed above, plus a few more.
1. Why the molecules of life are built around carbon
 Life is complex. Carbon allows for that complexity at the molecular level. Here’s why.
Life is complex. Carbon allows for that complexity at the molecular level. Here’s why.
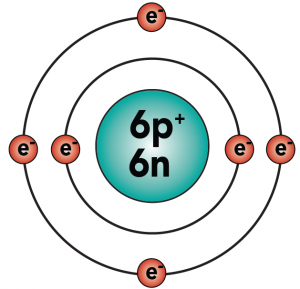
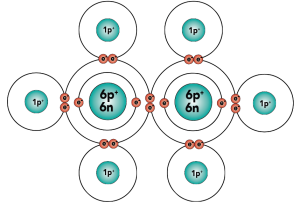
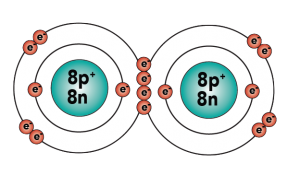
Carbon is element number six in the periodic table. It’s number six because it has six protons, along with six neutrons and six electrons.
With four outer (also known as valence) electrons, carbon readily forms covalent bonds with other atoms, including other carbon atoms.
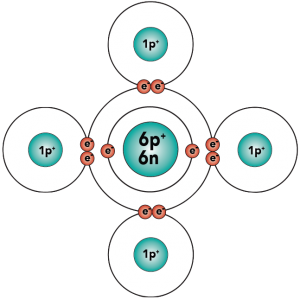
Electron distribution model |
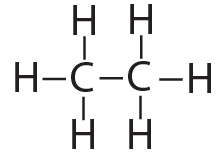
structural formula |
 |
 |
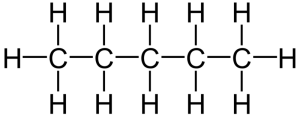
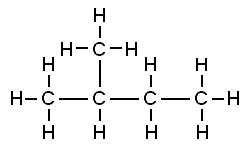
That carbon-carbon sharing makes it possible for carbon to form chains, branched molecules, and rings.
| Chain | Branched molecules | Rings |
 |
 |
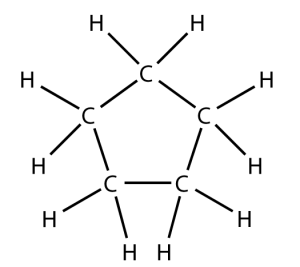 |
The possibilities for complexity and variety are further enhanced by carbon’s ability to form double bonds and triple bonds, as shown below…
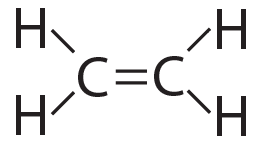
Ethylene (CH4) has a double bond |
Acetylene (CH2) has a triple bond |
So why is this important? The ability of carbon to form bonds with other atoms, particularly with itself, sets the stage for the evolution of the complex molecules that make life possible. Living things do phenomenally complex things, such as taking in matter and energy from the environment to grow and develop, and then reproducing and passing on genes to the next generation. On the molecular level, a variety of “molecular machines” are required to carry out these tasks, and these machines are big molecules, built around a carbon backbone. The flexibility of carbon-based molecules allows for the complexity of life. In the next tutorial, we’ll start seeing how the molecules of life are constructed. But before that, let’s check to make sure that you’re clear on what’s above.
2. Checking Understanding: Carbon, the Central Element
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Carbon, The Central Element (2.0)”]
[h] Carbon: The Central Element
[i]
[q] Carbon has _____ protons
[textentry single_char=”true”]
[c]ID Y=[Qq]
[f]IFllcy4gQ2FyYm9uIGhhcyA2IHByb3RvbnMu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBCdXQgeW91IGNhbiBmaWd1cmUgaXQgb3V0IGJ5IGV4YW1pbmluZyB0aGlzIGRpYWdyYW0u[Qq]
[q] Carbon has _______ outer (or valence) electrons.
[textentry single_char=”true”]
[c]ID Q=[Qq]
[f]IFllcy4gQ2FyYm9uIGhhcyA0wqBvdXRlciAob3IgdmFsZW5jZSkgZWxlY3Ryb25zLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBCdXQgeW91IGNhbiBmaWd1cmUgaXQgb3V0IGJ5IGV4YW1pbmluZyB0aGlzIGRpYWdyYW0u
Cg==[Qq]
[q multiple_choice=”true”] In ethane, is the bond connecting the two carbons a single bond or a double bond?
[c]IHNpbm dsZQ==[Qq]
[f]IENvcnJlY3QuIFRoZSBib25kIGJldHdlZW4gdGhlIHR3byBjYXJib25zIChhbmQgYmV0d2VlbiB0aGUgY2FyYm9ucyBhbmQgdGhlIGh5ZHJvZ2VucykgaXMgYSBzaW5nbGUgYm9uZCwgaW52b2x2aW5nIGEgc2luZ2xlIHNoYXJlZCBwYWlyIG9mIGVsZWN0cm9ucy4=[Qq]
[c]IGRvdWJsZQ==[Qq]
[f]IE5vLiBXaGVuIGF0b21zIHNoYXJlIGEgc2luZ2xlIHBhaXIgb2YgZWxlY3Ryb25zLCBpdCYjODIxNztzIGEgc2luZ2xlIGJvbmQu[Qq]
[q]Because carbon has four outer electrons, it can be organized into a variety of forms. Label these forms in the table below.
| ______________ | _____________ | ____________ |
[l]branches
[f*] Good!
[fx] No. Please try again.
[l]chains
[f*] Great!
[fx] No. Please try again.
[l]rings
[f*] Excellent!
[fx] No. Please try again.
[q] In your own words, explain why carbon is the central element in living things.
[c]IFtzaG93X21lX3Bs YWNlaG9sZGVyXQ==[Qq]
[f]IENhcmJvbiBoYXMgZm91ciB2YWxlbmNlIGVsZWN0cm9ucy4gVGhlIHJlc3VsdGluZyBmbGV4aWJpbGl0eSBpbiBmb3JtaW5nIGJvbmRzIGVuYWJsZXMgY2FyYm9uLWJhc2VkIG1vbGVjdWxlcyB0byBiZSBsYXJnZSBhbmQgY29tcGxleCwgYW5kIGluY2x1ZGUgYnJhbmNoZXMsIHJpbmdzLCBhbmQgY2hhaW5zLiBUaGlzLCBpbiB0dXJuLCBjcmVhdGVzIHRoZSBraW5kIG9mIG1vbGVjdWxhciBzdHJ1Y3R1cmVzIHRoYXQgbWFrZSBsaWZlJiM4MjE3O3MgZXNzZW50aWFsIGZ1bmN0aW9ucyAoc3VjaCBhcyBlbmVyZ3kgdHJhbnNmZXIgYW5kIHN0b3JhZ2UsIGluZm9ybWF0aW9uIHN0b3JhZ2UsIHJlcHJvZHVjdGlvbiwgZXRjLikgcG9zc2libGUu[Qq]
[/qwiz]
3. Hydrogen, Nitrogen, Oxygen, Phosphorus, and Sulfur
Here’s what you need to know about the other key elements in living things.
3.1. Hydrogen
Hydrogen — which is by far the most abundant element in the universe — plays many key roles in living things. Here are just a few.
- It’s a part of water, the most abundant compound, by weight, in any living thing.
- It plays a key role in energy transfer. The simple sugar glucose (C6H12O6) is energy-rich because it has a lot of hydrogen.
- Hydrogen ions (or protons) are used to create acidic environments within certain parts of cells, or in organs like the stomach. Controlling the flow of protons is how cells generate a molecule called ATP, which cells use to perform work.
3.2. Nitrogen
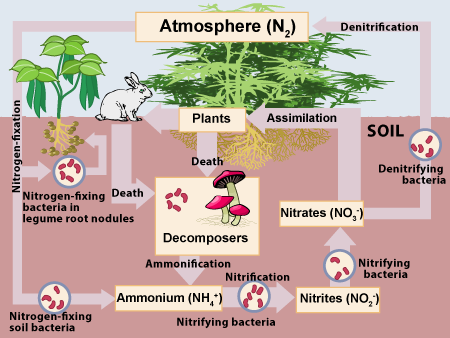
Nitrogen is a key part of proteins and nucleic acids like DNA and RNA. Nitrogen is also in ATP, life’s energy transfer molecule.
While nitrogen gas (N2) makes up most of the atmosphere (78%), few organisms can directly absorb nitrogen. The main organisms that do this are called nitrogen-fixing bacteria. These bacteria use energy, often provided by plants, to absorb nitrogen gas from the atmosphere and incorporate it into organic compounds like proteins, DNA and RNA. That’s the basis of the nitrogen cycle, shown at right.
3.3. Oxygen
Oxygen has several key roles.
- Along with hydrogen, it’s a part of water.
- It’s built into almost all biological molecules.
- It’s required for cellular respiration.
The Earth’s atmosphere is unique in that it contains a large proportion (about 21%) of free oxygen (O2). This oxygen is a planetary scale biogenic signature: a sign of life. That’s because free oxygen is generated by photosynthesis, during which photosynthetic organisms take water molecules and split them apart, releasing oxygen gas as a waste product. About 3.5 billion years ago, photosynthetic bacteria started releasing free O2 as a by-product of photosynthesis. Over the next few billion years, that oxygen saturated the oceans, and then bubbled out into the air. About 400 million years ago enough oxygen had formed to generate a layer of ozone (03). Ozone absorbs DNA-damaging ultraviolet radiation from the sun, so once enough oxygen had accumulated, life could leave the seas and colonize the land.
3.4. Phosphorus
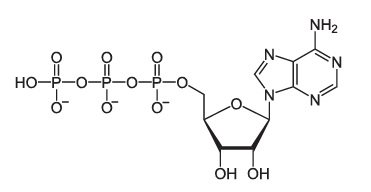
Phosphorus is the central atom in phosphate groups (-PO4), which we’ll meet in the next tutorial. Phosphate groups
- Play a key role in the structure of ATP.
- Are an essential part of DNA and RNA.
- Play a key role in the structure of phospholipids, the key structural molecules in cell membranes.
Because of phosphorus’ importance, it’s often a key limiting nutrient in ecosystems. On the other hand, pollution of waterways with phosphate, caused by runoff from farms or improperly treated wastewater, can cause blooms of algae that can harm or destroy aquatic ecosystems.
3.5. Sulfur
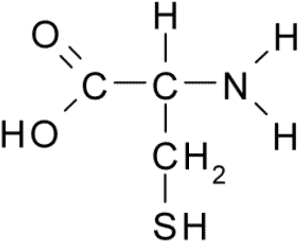
Sulfur is a key part of two of the twenty amino acids that make up proteins. Within proteins, bonds between sulfur atoms play a key role in determining protein shape, which in turn determines protein function.
4. Elements of Life: Checking Understanding
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Elements of Life F-I-B(2.0)”]
[h]Elements of Life: Checking Understanding
[i]
CHNOPS
[q]This gas is in our atmosphere because of photosynthesis.
[hangman]
[c]b3h5Z2Vu[Qq]
[q]The central atom in phosphate groups, which are found in ATP, DNA, and RNA.
[hangman]
[c]cGhvc3Bob3J1cw==[Qq]
[q]This gas makes up most of our atmosphere, but only a few bacteria can incorporate it into organic compounds.
[hangman]
[c]bml0cm9nZW4=[Qq]
[q]Glucose is energy-rich because of this element.
[hangman]
[c]aHlkcm9nZW4=[Qq]
[q]Found in two of the twenty amino acids that make up proteins, and required for protein structure.
[hangman]
[c]c3VsZnVy[Qq]
[q]The central element in living things.
[hangman]
[c]Y2FyYm9u[Qq]
[q]A six-letter acronym that you can use to remember the six key elements in living things.
[hangman]
[c]Q0hOT1BT[Qq]
[/qwiz]
What’s next?
-
- Continue to the next AP Bio tutorial: Functional Groups