1. Water, Life, and Gummy Bears
In the previous tutorial, we looked at how various substances and particles move across membranes. These substances, for the most part, were solutes in the cell’s watery cytoplasm or in the watery environment outside of the cell. But water itself is constantly moving in and out of cells, and that has enormous consequences. That’s why the water movement is the subject of this entire tutorial.
To start, instead of looking at cells or organisms, we’re going to look at gummy bears, because they’re a great experimental subject for demonstrating some introductory principles related to water flow.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Gummies in Water (2.0)”]
[h] Gummy bears in water
[q] Feel free to try this experiment at home. If you take a gummy bear and soak it in water for a few hours, what will happen?
[c]IFNob3cgbWUgdG hlIGFuc3dlcg==[Qq]
[f]IFRoZSByZWQgZ3VtbXkgYmVhciBoYXNuJiM4MjE3O3QgYmVlbiBzb2FraW5nIGluIHdhdGVyLiBUaGUgZ3JlZW4gZ3VtbXkgYmVhciBoYXMgYmVlbiBzb2FraW5nIGluIHdhdGVyIGZvciBzZXZlcmFsIGhvdXJzLiBBcyB5b3UgY2FuIHNlZSwgdGhlIGd1bW15IGJlYXIgaW4gd2F0ZXIgd2lsbCBpbmNyZWFzZSBpbiBzaXplLiBJZiB5b3Ugd2VpZ2hlZCBpdCwgeW91JiM4MjE3O2QgZmluZCB0aGF0IGl0cyBtYXNzIGluY3JlYXNlcyB1cCB0byBmaXZlIHRpbWVzLiA=V2hhdCYjODIxNztzIGdvaW5nIG9uPw==
Cg==[Qq]
[/qwiz]
2. Osmosis is water flow from hypotonic to hypertonic
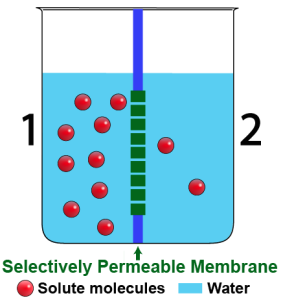
What you just observed is the results of osmosis. Osmosis is the diffusion of water. Just like anything else, water flows from higher concentration to lower concentration. But because it’s the solvent, this flow of water has many consequences.
Consider the diagram to the left. It’s a side view of a beaker that holds two solutions. The solutions are separated by a selectively permeable membrane. The membrane allows water to pass through, but not the solute, which is represented by the red spheres.
As you can see, there’s more solute on side 1 than on side 2. If there are more solute molecules, then there have to be fewer water molecules. Here’s a summary of the situation:
| Side 1 | Side 2 |
| A higher percentage of solute molecules | A lower percentage of solute molecules |
| A lower percentage of water molecules | A higher percentage of water molecules |
A somewhat outdated way to refer to a solute is as the tonic (a phrase we hold onto in phrases like “gin and tonic,” where the tonic is dissolved in the gin). Using that, we wind up with two more descriptions of the situation above:
- Side 1 is hypertonic to side 2. That means that the solution on side 1 has more solute dissolved in it than the solution on side 2.
- Side 2 is hypotonic to side 1. That means that the solution on side 2 has less solute dissolved in it than the solution on side 1.
You can remember this by focusing on the prefixes hypo and hyper.
- Hypo means under, or beneath, as in hypodermic (beneath the skin). Hypotonic means less solute.
- Hyper means too much, as in hyperactive (too active). Hypertonic means more solute.
Since water (like everything else) diffuses from higher concentration to lower concentration, we can expect that water will flow from side 2 to side 1. Another way to say that is that water will diffuse from the hypotonic side to the hypertonic side. As we’ll see in the next section, that will cause the water level within side 1 to rise.

That’s why the green gummy bear expanded. Water flowed from the water surrounding it into the gummy bear. Why? Because the water was hypotonic to the gummy bear (which was hypertonic to the water in which it was placed). To accommodate this inflow of water, the gummy expanded.
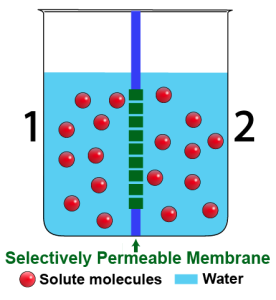
The solutions on opposite sides of a membrane can also have the same concentration of water, which is to say that they can have the same concentration of dissolved solute. The word for that is isotonic. Here’s a diagram of two solutions that are isotonic to one another.
SUMMARY:
- Osmosis is the diffusion of water.
- A hypertonic solution has relatively more dissolved solute than an adjacent solution.
- A hypotonic solution has relatively less dissolved solute than an adjacent solution.
- Isotonic solutions have equal amounts of dissolved solutes.
- Water always diffuses from hypotonic to hypertonic.
3. Osmosis Vocabulary Flashcards
[qdeck bold_text=”false” qrecord_id=”sciencemusicvideosMeister1961-Osmosis Vocabulary (2.0)”]
[h]Osmosis Vocabulary Flashcards
[i]Many of the following cards ask you to create definitions in terms of both solution concentration and water concentration. This is an important AP Bio skill. Work towards fluency in your responses.
[q]Define diffusion.
[a]Diffusion is the movement of molecules from higher to lower concentration. Another way to say this is that diffusion is the movement of a substance down its concentration gradient.
[q]Define osmosis.
[a]Osmosis is the diffusion of water.
[q]Using two imaginary solutions, “A” and “B,” define hypotonic 1) in terms of dissolved solute, and 2) in terms of water concentration.
[a]In terms of dissolved solute: if solution “A” has less dissolved solute than solution “B,” then solution “A” is hypotonic to solution “B.”
In terms of water concentration: If solution “A” has a higher water concentration than solution “B,” then solution “A” is hypotonic to solution “B.”
[q]Using two imaginary solutions, “A” and “B,” define hypertonic 1) in terms of dissolved solute, and 2 in terms of water concentration.
[a]In terms of dissolved solute, if solution “A” has more dissolved solute than solution “B,” then solution “A” is hypertonic to solution “B.”
In terms of water concentration, if solution “A” has a lower water concentration than solution “B,” then solution “A” is hypertonic to solution “B.”
[q]Using two imaginary solutions, “A” and “B,” define isotonic 1) in terms of dissolved solute, and 2) in terms of water concentration.
[a]In terms of dissolved solute: If solution “A” has the same amount of dissolved solute as solution “B,” then solution “A” is isotonic to solution “B.”
In terms of water concentration: if solution “A” has the same water concentration as solution “B,” then solution “A” is isotonic to solution “B.”
[q]Using the terms hypotonic and hypertonic, describe how water flows.
[a]Water always flows from hypotonic to hypertonic.
[/qdeck]
4. Osmosis Vocabulary Fill-in-the-Blanks Quiz
Some of these questions test you on topics related to diffusion from the previous topic. Others review topics related to solutions (solute, solvent, etc.)
[qwiz random = “true” qrecord_id=”sciencemusicvideosMeister1961-Osmosis FIB (2.0)”]
[h]Osmosis Vocabulary Quiz
[i]
[q]The movement of a substance from higher concentration to lower concentration is [hangman].
[c]ZGlmZnVzaW9u
Cg==[Qq]
[q]The diffusion of water is [hangman].
[c]b3Ntb3Npcw==
Cg==[Qq]
[q]A mixture in which one thing is dissolved in another is a(n) [hangman].
[c]c29sdXRpb24=
Cg==[Qq]
[q]In a solution, the thing that does the dissolving is the [hangman].
[c]c29sdmVudA==[Qq]
[q]In a solution, the thing that gets dissolved is the [hangman].
[c]c29sdXRl[Qq]
[q]In lemonade (that you make from a mix), the lemonade mix is the [hangman].
[c]c29sdXRl[Qq]
[q]In lemonade (that you make from a mix), the water is the [hangman].
[c]c29sdmVudA==[Qq]
[q]Solution “A” has more dissolved solute than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwZXJ0b25pYw==[Qq]
[q]Solution “A” has less dissolved solute than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwb3Rvbmlj
Cg==[Qq]
[q]Solution “A” has the same amount of dissolved solute as solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aXNvdG9uaWM=[Qq]
[q]Solution “A” has a higher percentage of water than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwb3Rvbmlj[Qq]
[q]Solution “A” has a lower percentage of water than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwZXJ0b25pYw==[Qq]
[q]If I want to make a solution more hypertonic, I add more [hangman].
[c]c29sdXRl[Qq]
[q]If I want to make a solution more hypotonic, I add more [hangman].
[c]c29sdmVudA==[Qq]
[q]If I want to make a solution less hypertonic, I add more [hangman].
[c]c29sdmVudA==[Qq]
[x]
If you want more practice, please press the button below. Otherwise, follow the links below.
[restart]
[/qwiz]
5. Osmotic Pressure
The movement of water caused by osmosis generates a force called osmotic pressure. Osmotic pressure explains why our gummy bear expanded. Let’s look at it in a bit more detail.
| Original Situation | After Osmosis |
 |
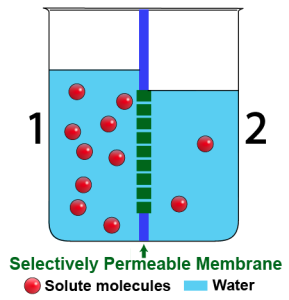 |
The beakers above show a time sequence. The beaker on the left shows the situation at the start: side 2 is hypotonic to side 1. The membrane is permeable to water, but not to the solution.
Over time, water will move, by osmosis, from side 2 into side 1. With more water molecules on side 1, the water level has to rise. With fewer water molecules on side 2, the water level there has to fall. You can see the result in the beaker on the right.
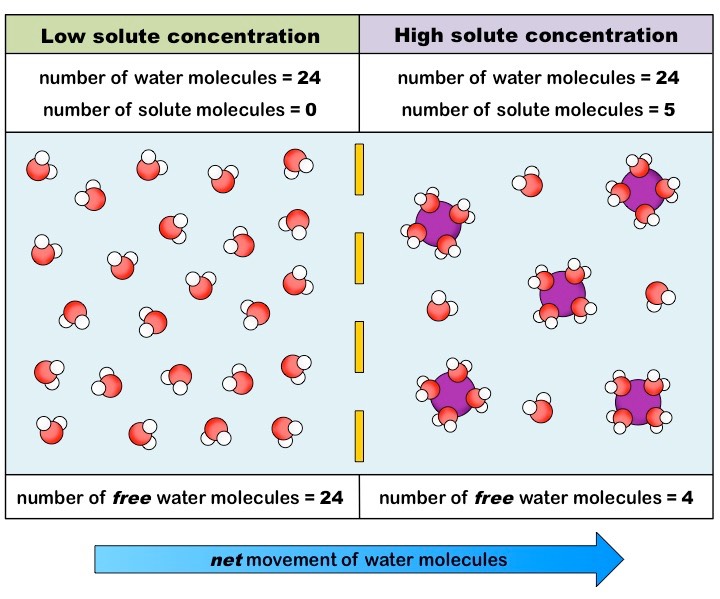
Students typically describe what’s happening in a situation like the one above by saying “water wants to move from the hypotonic side (side 2) to the hypertonic side (side 1).” But there’s a better way to explain what’s happening. The water molecules are randomly moving in both directions across the membrane. However, once the water molecules diffuse into the hypertonic side, they’ll interact with the solute in a way that keeps them from returning.
You can see this portrayed in the diagram on the right, On the left side, all the water molecules are free. On the right side, water molecules are interacting with the solute. Based on the way the water molecules are oriented around the solute molecules, you can assume that the solute particles are positively charged (because the negatively charged oxygen side of the water molecule is binding with it, and opposites attract. As a result, water molecules become stuck on the right side (because the membrane is impermeable to the solute).
6. Animal Cells and Osmosis
Let’s see how this can affect cells in hypotonic, isotonic, or hypertonic solutions.
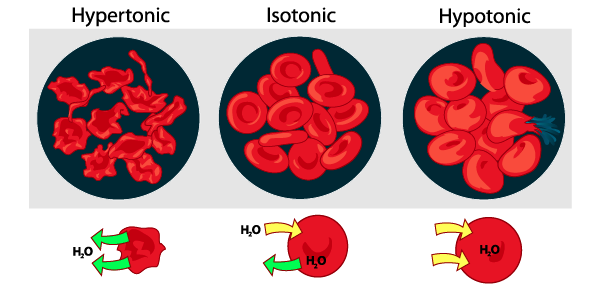
Consider the red blood cells shown above.
The cells in the hypotonic solution (on the right side of the diagram) are expanding and bursting. Why?
These cells were hypertonic to the surrounding solution. Because water flows from hypotonic to hypertonic, water will flow into the cells. This flow of water exerts pressure, much like the force you exert when you blow up a balloon. In the same way that a balloon expands, so will the cell, right up to the point where it bursts. In the same way that air pressure can burst a balloon, osmotic pressure can burst a red blood cell.
By contrast, the cells in the hypertonic solution (the one on the left) are shriveled. Why? Because water flows from hypotonic to hypertonic. If the cells are in a hypertonic solution, water will leave the cells, and flow into the solution. The loss of water causes the cells to shrivel up, just like the loss of water from a grape causes it to become a shriveled raisin. In the same way that loss of air pressure causes a tire to become flat (deflated), loss of osmotic pressure can cause a cell (and living tissues made of cells) to become shriveled.
In an isotonic solution (shown in the middle), water flows equally between the cells and the solution. The cell doesn’t gain water or lose water (because water diffuses into and out of it at equal rates).
7. Plant Cells and Osmosis
Because plant cells have a rigid wall, they respond to osmotic pressure differently.
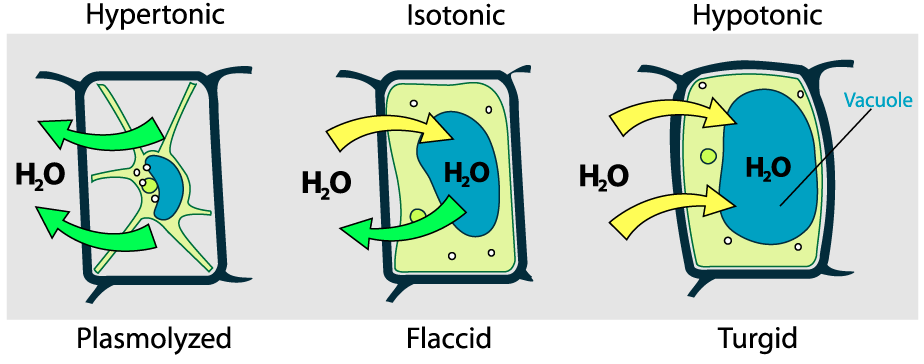
- In a hypertonic solution, a plant cell will lose water. As it does, the central vacuole contracts. This causes the cell membrane to peel away from the wall, a condition called plasmolysis. As a result, the plant as a whole becomes wilted and droopy.
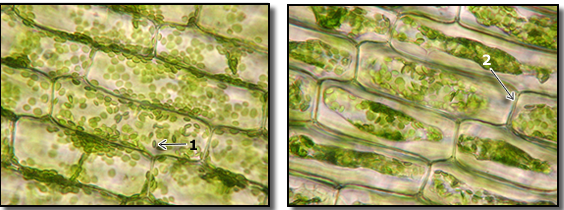
You can see what plasmolysis looks like on a microscopic level in the image on the right above. This is from an aquatic plant called Elodea. Elodea doesn’t have a central vacuole, but the effect is the same. Notice how in the micrograph on the left Elodea’s chloroplasts fill the entire volume of the cell. These cells are in spring water (a hypotonic solution). The cells on the right are in a solution of spring water with 10% salt. Because that’s hypertonic to the cells’ cytoplasm, water leaves the cells. As it does, the membrane peels away from the cell wall, concentrating the chloroplasts in the center of the cell.
- In a hypotonic solution, water will move into the plant cell. This will cause the central vacuole to expand. As it does, the entire cell will expand (just as the red blood cells did above). However, unlike red blood cells in a hypotonic solution, these plant cells won’t burst. Instead, the membrane will be pushed against the wall, keeping the plant cell solid and firm. A plant cell that’s full and firm because of the force of osmosis is said to be turgid, and the force of water pushing out against the wall is called turgor pressure.
This is a healthy condition for plant cells and plants as a whole. It’s why grocery stores use misters to keep their vegetables covered with water (a hypotonic solution), causing water to move into the veggies to keep them crisp and firm.
 |
 |
| Wilted lettuce, caused by loss of osmotic pressure | A grocery mister. The hypotonic water keeps the veggies crisp. |
8. Contractile Vacuoles and Osmosis
In the examples above, we’ve seen the consequences of osmosis on animal and plant cells as water flows into them our out of them. But organisms can also regulate the flow of water, a process that’s called osmoregulation.
We’ll look at a variety of osmoregulatory adaptations in Unit 4 when we explore homeostasis. But for now, let’s consider the contractile vacuole found in freshwater protists. Protists are unicellular eukaryotes. The three shown here are common in lakes and streams (and are commonly viewed in an AP Biology laboratory).
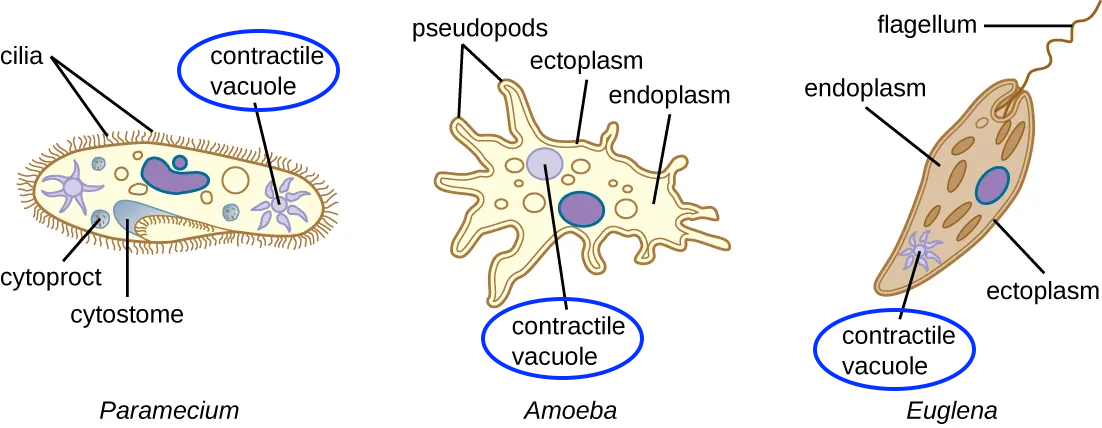
Protists that live in freshwater share a common osmoregulatory challenge. Because their cytoplasm is hypertonic to their freshwater environment, water moves into these cells by osmosis. How do they keep from bursting, like the animal cell shown above? To osmoregulate, these protists contain an organelle called a contractile vacuole. This is a pump: osmosis fills the contractile vacuole with water. Then, in a process that requires energy, the vacuole contracts and expels water from the cell. If the environment becomes more hypertonic (diminishing the water potential gradient) the cell can adapt by decreasing its rate of contractile vacuole contraction, and do the reverse in more hypotonic environments.
9. Osmosis Interactive Diagrams
[qwiz style = “border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Osmosis Interactive Diagrams (2.0)”]
[h] Osmosis Interactive Diagrams
[i]This activity tests your understanding of diagrams related to osmosis.
[q labels = “top”]
[l]selectively permeable membrane
[fx] No. Please try again.
[f*] Correct!
[l]hypertonic side
[fx] No, thatís not correct. Please try again.
[f*] Good!
[l]hypotonic side
[fx] No. Please try again.
[f*] Good!
[l]solute molecule
[fx] No. Please try again.
[f*] Great!
[l]water
[fx] No. Please try again.
[f*] Great!
[q labels = “top”]A gummy bear is put into water. Gummy bears are full of sugar. Label this osmotic situation.
[l]hypotonic
[fx] No, thatís not correct. Please try again.
[f*] Correct!
[l]hypertonic
[fx] No. Please try again.
[f*] Correct!
[q labels= “top”]Label this osmotic situation.
[l]hypotonic
[fx] No, thatís not correct. Please try again.
[f*] Good!
[l]hypertonic
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]Label this osmotic situation
[l]hypotonic
[fx] No. Please try again.
[f*] Good!
[l]hypertonic
[fx] No, thatís not correct. Please try again.
[f*] Great!
[q labels = “top”]Label the osmotic situation of the cell below, which is 95% water.
[l]hypertonic
[fx] No, thatís not correct. Please try again.
[f*] Correct!
[l]hypotonic
[fx] No, thatís not correct. Please try again.
[f*] Excellent!
[q labels = “top”]Label the osmotic situation of the cell below, which is 98% water.
[l]hypotonic
[fx] No, thatís not correct. Please try again.
[f*] Great!
[l]hypertonic
[fx] No, thatís not correct. Please try again.
[f*] Good!
[q labels = “top”]Label the osmotic situation of the cell below, which is 98% water.
[l]hypertonic
[fx] No, thatís not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No. Please try again.
[f*] Great!
[q labels = “top”]Drag the labels to complete the sentence: the cell is in a(n) ______ environment.
[l]isotonic
[fx] No, thatís not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No. Please try again.
[f*] Great!
[l]hypertonic
[fx] No. Please try again.
[f*] Good!
[q labels = “top”]Drag the labels to complete the sentence: the cell is in a(n) ______ environment.
[l]isotonic
[fx] No, that is not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No, that is not correct. Please try again.
[f*] Excellent!
[l]hypertonic
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]Drag the labels to complete the sentence: the cell is ______ to its environment.
[l]isotonic
[fx] No, that is not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No. Please try again.
[f*] Great!
[l]hypertonic
[fx] No. Please try again.
[f*] Good!
[q labels = “top”]Drag the labels to complete the sentence: the cell is ______ to its environment.
[l]isotonic
[fx] No, that is not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No, that is not correct. Please try again.
[f*] Excellent!
[l]hypertonic
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]Drag the labels as if you were completing the sentence “These elodea cells are ____________ to their environment.”
[l]hypotonic
[fx] No. Please try again.
[f*] Correct!
[l]hypertonic
[fx] No. Please try again.
[f*] Good!
[q labels = “top”]Drag the labels as if you were completing the sentence “These elodea cells are in a(n) ____________ environment.”
[l]hypotonic
[fx] No. Please try again.
[f*] Correct!
[l]hypertonic
[fx] No. Please try again.
[f*] Good!
[q]Label the parts of these elodea cells. The cells start in fresh water and then are exposed to salt water.
[l]cell membrane
[fx] No. Please try again.
[f*] Correct!
[l]cell wall
[fx] No. Please try again.
[f*] Excellent!
[l]chloroplast
[fx] No, thatís not correct. Please try again.
[f*] Correct!
[x]
[restart]
[/qwiz]
10. Osmosis Quiz 2
[qwiz style = “border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Osmosis Quiz 2 (2.0)”]
[h]Osmosis Quiz 2
[i]This quiz tests you on your understanding of osmosis, and your ability to explain phenomena like the one below.
[q]A solution that has a higher solute concentration than a solution on the other side of the membrane is ___________
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7IFdoYXQgcHJlZml4IGlzIGFzc29jaWF0ZWQgd2l0aCAmIzgyMjA7bW9yZSYjODIyMTsgb3IgJiM4MjIwO3RvbyBtdWNoLiYjODIyMTs=[Qq]
[f]RXhjZWxsZW50LsKgQSBzb2x1dGlvbiB0aGF0IGhhcyBhIGhpZ2hlciBzb2x1dGUgY29uY2VudHJhdGlvbiB0aGFuIGEgc29sdXRpb24gb24gdGhlIG90aGVyIHNpZGUgb2YgdGhlIG1lbWJyYW5lIGlzwqA=aHlwZXJ0b25pYw==Lg==[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDt0aGUgc2FtZSBhbW91bnQgb2Ygc29sdXRlLiYjODIyMTsgV2hhdCBwcmVmaXggaXMgYXNzb2NpYXRlZCB3aXRoICYjODIyMDttb3JlJiM4MjIxOyBvciAmIzgyMjA7dG9vIG11Y2guJiM4MjIxOw==
Cg==[Qq]
[q]A solution that has a higher water concentration than a solution on the other side of the membrane is ___________
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiA=SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBMZXNzIHNvbHV0ZSBtZWFuc8KgaGlnaGVyIHdhdGVyIGNvbmNlbnRyYXRpb24u[Qq]
[f]Tm8uIEh5cGVydG9uaWMgbWVhbnMgaGlnaGVyIA==c29sdXRlIGNvbmNlbnRyYXRpb24uwqBJZiB0aGVyZSYjODIxNztzIG1vcmUgc29sdXRlLCB0aGVyZSBoYXMgdG8gYmUgbGVzcyB3YXRlci4gWW91JiM4MjE3O3JlIGxvb2tpbmcgZm9yIHRoZSB0ZXJtIHRoYXQgbWVhbnMgJiM4MjIwO2xvd2VyIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTs=[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSAob3Igd2F0ZXIpIGNvbmNlbnRyYXRpb24uJiM4MjIxO8KgSGlnaGVyIHdhdGVyIGNvbmNlbnRyYXRpb24gbWVhbnMgJiM4MjIwO2xvd2VyIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTsgV2hpY2ggdGVybSBtZWFuc8KgJiM4MjIwO2xvd2VyIHNvbHV0ZSBjb25jZW50cmF0aW9uPyYjODIyMTs=
Cg==[Qq]
[q]A solution that has the same solute concentration as a solution on the other side of the membrane is ___________
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG 9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3IgYSB0ZXJtwqB0aGF0IG1lYW5zICYjODIyMDt0aGUgc2FtZSBzb2x1dGXCoGNvbmNlbnRyYXRpb24uJiM4MjIxOyBXaGF0IHByZWZpeCBtZWFucyAmIzgyMjA7dGhlIHNhbWU/JiM4MjIxOyBUaGluayBvZiBhIHRyaWFuZ2xlIHRoYXQgaGFzIHR3byBzaWRlcyB0aGF0IGFyZSB0aGUgc2FtZSYjODIzMDs=[Qq]
[f]Tm8uIEh5cGVydG9uaWMgbWVhbnMgaGlnaGVyIA==c29sdXRlIGNvbmNlbnRyYXRpb24uwqBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3IgYSB0ZXJtwqB0aGF0IG1lYW5zICYjODIyMDt0aGUgc2FtZSBzb2x1dGXCoGNvbmNlbnRyYXRpb24uICYjODIyMDtXaGF0IHByZWZpeCBtZWFucyAmIzgyMjA7dGhlIHNhbWU/JiM4MjIxOyBUaGluayBvZiBhIHRyaWFuZ2xlIHRoYXQgaGFzIHR3byBzaWRlcyB0aGF0IGFyZSB0aGUgc2FtZSYjODIzMDs=[Qq]
[f]WWVzIcKgSXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTsgSnVzdCByZW1lbWJlciB0aGUgaXNvc2NlbGVzIHRyaWFuZ2xlLg==
Cg==[Qq]
[q]A solution that has a lower solute concentration than a solution on the other side of the membrane is ___________
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiA=SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7[Qq]
[f]Tm8uIA==SHlwZXJ0b25pYw==IG1lYW5zIA==aGlnaGVyIHNvbHV0ZSBjb25jZW50cmF0aW9uLiBUaGUgdGVybSB0aGF0IG1lYW5zIA==[Qq]lower solute concentration begins with a prefix that means “lower.”
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDt0aGUgc2FtZSBhbW91bnQgb2Ygc29sdXRlLiYjODIyMTsgVGhlIHRlcm0gdGhhdCBtZWFucyA=bG93ZXIgc29sdXRlIGNvbmNlbnRyYXRpb24=IGJlZ2lucyB3aXRoIGEgcHJlZml4IHRoYXQgbWVhbnMgJiM4MjIwO2xvd2VyLiYjODIyMTs=
[Qq][q]A solution that has a lower water concentration than a solution on the other side of the membrane is ___________
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBMZXNzIHNvbHV0ZSBtZWFuc8KgaGlnaGVyIHdhdGVyIGNvbmNlbnRyYXRpb24uIFdoYXQgdGVybSBtZWFucyAmIzgyMjA7aGlnaGVyIHNvbHV0ZSBjb25jZW50cmF0aW9uPyYjODIyMTs=[Qq]
[f]WWVzLiBIeXBlcnRvbmljIG1lYW5zIGhpZ2hlciA=c29sdXRlIGNvbmNlbnRyYXRpb24uwqBJZiB0aGVyZSYjODIxNztzIG1vcmUgc29sdXRlLCB0aGVyZSBoYXMgdG8gYmUgbGVzcyB3YXRlci4=[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSAob3Igd2F0ZXIpIGNvbmNlbnRyYXRpb24uJiM4MjIxOyBMb3dlcsKgd2F0ZXIgY29uY2VudHJhdGlvbiBtZWFucyAmIzgyMjA7aGlnaGVyIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTsgV2hpY2ggdGVybSBtZWFuc8KgJiM4MjIwO2hpZ2hlciBzb2x1dGUgY29uY2VudHJhdGlvbj8mIzgyMjE7
Cg==[Qq]
[q]A solution that has the same water concentration as a solution on the other side of the membrane is ___________
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG 9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3IgYSB0ZXJtwqB0aGF0IG1lYW5zICYjODIyMDt0aGUgc2FtZSBzb2x1dGXCoGNvbmNlbnRyYXRpb24mIzgyMjE7IChiZWNhdXNlIGlmIHRoZSBzb2x1dGUgY29uY2VudHJhdGlvbiBpcyB0aGUgc2FtZSwgdGhlIHdhdGVyIGNvbmNlbnRyYXRpb24gYWxzbyBoYXMgdG8gYmUgdGhlIHNhbWUpLiBXaGF0IHByZWZpeCBtZWFucyAmIzgyMjA7dGhlIHNhbWU/JiM4MjIxOyBUaGluayBvZiBhIHRyaWFuZ2xlIHRoYXQgaGFzIHR3byBzaWRlcyB0aGF0IGFyZSB0aGUgc2FtZSYjODIzMDs=[Qq]
[f]Tm8uIEh5cGVydG9uaWMgbWVhbnMgaGlnaGVyIA==c29sdXRlIGNvbmNlbnRyYXRpb24uwqBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3IgYSB0ZXJtwqB0aGF0IG1lYW5zICYjODIyMDt0aGUgc2FtZSBzb2x1dGXCoGNvbmNlbnRyYXRpb24mIzgyMjE7IChiZWNhdXNlIGlmIHRoZSBzb2x1dGUgY29uY2VudHJhdGlvbiBpcyB0aGUgc2FtZSwgdGhlIHdhdGVyIGNvbmNlbnRyYXRpb24gYWxzbyBoYXMgdG8gYmUgdGhlIHNhbWUpLiBXaGF0IHByZWZpeCBtZWFucyAmIzgyMjA7dGhlIHNhbWU/JiM4MjIxOyBUaGluayBvZiBhIHRyaWFuZ2xlIHRoYXQgaGFzIHR3byBzaWRlcyB0aGF0IGFyZSB0aGUgc2FtZSYjODIzMDs=[Qq]
[f]WWVzIcKgSXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTvCoElmIHRoZSBzb2x1dGUgY29uY2VudHJhdGlvbiBpcyB0aGUgc2FtZSwgdGhlIHdhdGVyIGNvbmNlbnRyYXRpb24gd2lsbCBhbHNvIGJlIHRoZSBzYW1lLg==
Cg==[Qq]
[q]A gummy bear is placed in water. The gummy bear is made mostly of sugar, held together by gelatin. The gummy bear is ___________ to the water.
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBZb3UmIzgyMTc7cmUgY29tcGFyaW5nIGEgZ3VtbXkgYmVhciwgd2l0aCBhbGwgb2YgaXRzIHN1Z2FyLCB0byBtb3N0bHkgcHVyZSB3YXRlci4gV2hhdCB3b3JkIG1lYW5zICYjODIyMDttb3JlIHNvbHV0ZT8mIzgyMjE7[Qq]
[f]RXhjZWxsZW50LsKgQSBndW1teSBiZWFyLCB3aXRoIGFsbCBvZiBpdHMgc3VnYXIswqBoYXMgYSBoaWdoZXIgc29sdXRlIGNvbmNlbnRyYXRpb24gdGhhbiB0aGUgd2F0ZXIgdGhhdCBpdCYjODIxNztzIGluLiDCoFRoYXQgbWFrZXMgdGhlIGd1bW15wqA=aHlwZXJ0b25pYw==IHRvIHRoZSB3YXRlci4=[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDt0aGUgc2FtZSBhbW91bnQgb2Ygc29sdXRlLiYjODIyMTsgWW91JiM4MjE3O3JlIGNvbXBhcmluZyBhIGd1bW15IGJlYXIsIHdpdGggYWxsIG9mIGl0cyBzdWdhciwgdG8gbW9zdGx5IHB1cmUgd2F0ZXIuIFdoYXQgd29yZCBtZWFucyAmIzgyMjA7bW9yZSBzb2x1dGU/JiM4MjIxOw==
Cg==[Qq]
[q]In this diagram, the cell is ________ to the solution outside the cell
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7IExvb2sgYXQgaG93IG11Y2ggbW9yZSBzb2x1dGUgaXMgaW5zaWRlIHRoZSBjZWxsIHRoYW4gb3V0c2lkZS4gV2hhdCB0ZXJtIG1lYW5zICYjODIyMDtoaWdoZXIgc29sdXRlIGNvbmNlbnRyYXRpb24/JiM4MjIxOw==[Qq]
[f]WWVzLiBIeXBlcnRvbmljIG1lYW5zIGhpZ2hlciA=c29sdXRlIGNvbmNlbnRyYXRpb24uIEFzIHlvdSBjYW4gc2VlLCB0aGUgY2VsbCBpcyBoeXBlcnRvbmljIHRvIGl0cyBlbnZpcm9ubWVudC4=[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSAob3Igd2F0ZXIpIGNvbmNlbnRyYXRpb24uJiM4MjIxO8KgTG9vayBhdCBob3cgbXVjaCBtb3JlIHNvbHV0ZSBpcyBpbnNpZGUgdGhlIGNlbGwgdGhhbiBvdXRzaWRlLiBXaGF0IHRlcm0gbWVhbnMgJiM4MjIwO2hpZ2hlciBzb2x1dGUgY29uY2VudHJhdGlvbj8mIzgyMjE7
Cg==[Qq]
[q]In this diagram, the cell is ________ to the solution outside the cell
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiA=SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7IEFzIHlvdSBjYW4gc2VlLCB0aGUgY2VsbCBpcyBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50Lg==[Qq]
[f]Tm8uIEh5cGVydG9uaWMgbWVhbnMgaGlnaGVyIA==c29sdXRlIGNvbmNlbnRyYXRpb24uIEp1c3QgYnkgbG9va2luZywgeW91IGNhbiB0ZWxsIHRoYXQgdGhlcmUmIzgyMTc7cyBhIGxvd2VyIGNvbmNlbnRyYXRpb24gb2Ygc29sdXRlIGluc2lkZSB0aGUgY2VsbCB0aGFuIG91dHNpZGUgdGhlIGNlbGwuIMKgV2hhdCB0ZXJtIG1lYW5zICYjODIyMDtsb3dlciBzb2x1dGUgY29uY2VudHJhdGlvbj8mIzgyMjE7[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSAob3Igd2F0ZXIpIGNvbmNlbnRyYXRpb24uJiM4MjIxO8KgSnVzdCBieSBsb29raW5nLCB5b3UgY2FuIHRlbGwgdGhhdCB0aGVyZSYjODIxNztzIGEgbG93ZXIgY29uY2VudHJhdGlvbiBvZiBzb2x1dGUgaW5zaWRlIHRoZSBjZWxsIHRoYW4gb3V0c2lkZSB0aGUgY2VsbC4gwqBXaGF0IHRlcm0gbWVhbnMgJiM4MjIwO2xvd2VyIHNvbHV0ZSBjb25jZW50cmF0aW9uPyYjODIyMTs=
Cg==[Qq]
[q]In this situation, water will
[c]ZmxvdyBpbnRvIH RoZSBjZWxsLg==[Qq]
[c]ZmxvdyBvdXQgb2YgdGhlIGNlbGw=[Qq]
[c]ZmxvdyBpbnRvIGFuZCBvdXQgb2YgdGhlIGNlbGwgYXQgZXF1YWwgcmF0ZXMsIHdpdGggbm8gbmV0IGNoYW5nZS4=[Qq]
[f]WWVzLiBXYXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYy7CoEJlY2F1c2UgdGhlIGNlbGwgaXMgaHlwZXJ0b25pYyB0byBpdHMgZW52aXJvbm1lbnQsIHdhdGVyIHdpbGwgZmxvdyA=aW50bw==IHRoZSBjZWxsLg==[Qq]
[f]Tm8uIFdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLiBJcyB0aGlzIGNlbGwgaHlwZXJ0b25pYyBvciBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50Pw==[Qq]
[f]Tm8uIFdhdGVyIGZsb3cgd291bGQgYmUgZXF1YWwgaWYgdGhlIGNlbGwgd2VyZSBpc290b25pYyB0byBpdHMgZW52aXJvbm1lbnQsIGFuZCB0aGF0JiM4MjE3O3Mgbm90IHRoZSBjYXNlLiBLZWVwIGluIG1pbmQgdGhhdCB3YXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYywgYW5kIGZpZ3VyZSBvdXQgdGhlIGFuc3dlci4=
Cg==[Qq]
[q]In this situation, water will
[c]ZmxvdyBpbnRvIHRoZSBjZWxsLg==[Qq]
[c]ZmxvdyBvdXQgb2 YgdGhlIGNlbGw=[Qq]
[c]ZmxvdyBpbnRvIGFuZCBvdXQgb2YgdGhlIGNlbGwgYXQgZXF1YWwgcmF0ZXMsIHdpdGggbm8gbmV0IGNoYW5nZS4=[Qq]
[f]Tm8uIFdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLiBUaGUgaHlwb3RvbmljIHNpZGUgaXMgdGhlIG9uZSB3aXRoIGxlc3Mgc29sdXRlLiBOb3cgZmlndXJlIG91dCB0aGUgYW5zd2VyLg==[Qq]
[f]WWVzLiBXYXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYy4gQmVjYXVzZSB0aGUgY2VsbCBpcyBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50LCB3YXRlciB3aWxsIGZsb3cgb3V0IG9mwqB0aGUgY2VsbC4=[Qq]
[f]Tm8uIFdhdGVyIGZsb3cgd291bGQgYmUgZXF1YWwgaWYgdGhlIGNlbGwgd2VyZSBpc290b25pYyB0byBpdHMgZW52aXJvbm1lbnQsIGFuZCB0aGF0JiM4MjE3O3Mgbm90IHRoZSBjYXNlLiBLZWVwIGluIG1pbmQgdGhhdCB3YXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYywgYW5kIGZpZ3VyZSBvdXQgdGhlIGFuc3dlci4=
Cg==[Qq]
[q]Assume that this cell is an animal cell (without a cell wall). In this situation, the cell will
[c]aW5jcmVhc2Ug aW4gc2l6ZQ==[Qq]
[c]ZGVjcmVhc2UgaW4gc2l6ZQ==[Qq]
[c]cmVtYWluIHRoZSBzYW1lIHNpemUu[Qq]
[f]WWVzLiBXYXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYy7CoEFzIHdhdGVyIGZsb3dzIGludG8gdGhlIGh5cGVydG9uaWMgY2VsbCwgaXQgd2lsbCBpbmNyZWFzZSBpbiBzaXplLg==[Qq]
[f]Tm8uIFdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLiBJcyB0aGlzIGNlbGwgaHlwZXJ0b25pYyBvciBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50PyBGaWd1cmUgdGhhdCBvdXQsIHRoZW4gZmlndXJlIG91dCB0aGUgZGlyZWN0aW9uIG9mIHdhdGVyIGZsb3cu[Qq]
[f]Tm8uIFRoZSBjZWxsIHdvdWxkIHN0YXkgdGhlIHNhbWUgc2l6ZSBpZiBpdCB3ZXJlIGlzb3RvbmljIHRvIGl0cyBlbnZpcm9ubWVudC4gSXQmIzgyMTc7cyBub3QuIEtlZXAgaW4gbWluZCB0aGF0IHdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLCBhbmQgZmlndXJlIG91dCB0aGUgYW5zd2VyLg==
Cg==[Qq]
[q]Assume that this cell is an animal cell (without a cell wall). In this situation, the cell will
[c]aW5jcmVhc2UgaW4gc2l6ZQ==[Qq]
[c]ZGVjcmVhc2Ug aW4gc2l6ZQ==[Qq]
[c]cmVtYWluIHRoZSBzYW1lIHNpemUu[Qq]
[f]Tm8uIFdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLiBJcyB0aGlzIGNlbGwgaHlwZXJ0b25pYyBvciBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50PyBGaWd1cmUgdGhhdCBvdXQsIHRoZW4gZmlndXJlIG91dCB0aGUgZGlyZWN0aW9uIG9mIHdhdGVyIGZsb3cu[Qq]
[f]WWVzLiBXYXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYy7CoEFzIHdhdGVyIGZsb3dzIG91dCBvZsKgdGhlIGh5cG90b25pY8KgY2VsbCwgaXQgd2lsbCBkZWNyZWFzZcKgaW4gc2l6ZS4=[Qq]
[f]Tm8uIFRoZSBjZWxsIHdvdWxkIHN0YXkgdGhlIHNhbWUgc2l6ZSBpZiBpdCB3ZXJlIGlzb3RvbmljIHRvIGl0cyBlbnZpcm9ubWVudC4gSXQmIzgyMTc7cyBub3QuIEtlZXAgaW4gbWluZCB0aGF0IHdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLCBhbmQgZmlndXJlIG91dCB0aGUgYW5zd2VyLg==
Cg==[Qq]
[q]When plant cells are in this environment, they’ll be the most solid and firm.
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiBXaGVuIHBsYW50IGNlbGxzIGFyZSBpbiBhIGh5cG90b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgZmxvd3MgaW50byB0aGUgY2VsbHMuIFRoaXMgZXhwYW5kcyB0aGUgY2VudHJhbCB2YWN1b2xlIGFuZCBwdXNoZXMgdGhlIG1lbWJyYW5lIGFnYWluc3QgdGhlIHdhbGwuIFRoYXQgbWFrZXMgdGhlIGNlbGxzIChhbmQgdGhlIGVudGlyZSBwbGFudCkgc29saWQgYW5kIGZpcm0u
Cg==[Qq]
[f]Tm8uIFdoZW4gcGxhbnQgY2VsbHMgYXJlIGluIGEgaHlwZXJ0b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgZmxvd3Mgb3V0IG9mIHRoZSBjZWxscy4gVGhpcyBjb250cmFjdHMgdGhlIGNlbnRyYWwgdmFjdW9sZSBhbmQgcGVlbHMgdGhlIG1lbWJyYW5lIGF3YXkgZnJvbSB0aGUgd2FsbC4gVGhhdCBtYWtlcyB0aGUgY2VsbHMgKGFuZCB0aGUgZW50aXJlIHBsYW50KSBkcm9vcHkgYW5kIHdpbHRlZC4=
Cg==[Qq]
[f]Tm8gKGJ1dCB0aGlzIGlzIHRoZSBzZWNvbmQtYmVzdCBhbnN3ZXIpLiBJZiBwbGFudCBjZWxscyBhcmUgaW4gYW4gaXNvdG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIHdpbGwgYmUgZW50ZXJpbmcgYW5kIGxlYXZpbmcgdGhlbSBhdCB0aGUgc2FtZSByYXRlLiBUaGUgY2VsbHMgd2lsbCBiZSBpbiByZWFzb25hYmx5IGdvb2Qgc2hhcGUsIGJ1dCBhIGNoYW5nZSBpbiB0aGVpciBvc21vdGljIGNvbmRpdGlvbiB3b3VsZCBtYWtlIHRoZSBwbGFudCBldmVuIG1vcmUgc29saWQgYW5kIGZpcm0u
Cg==Cg==[Qq]
[q]When animal cells are in this environment, they’ll shrink and shrivel
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIFdoZW4gYW5pbWFsIGNlbGxzIGFyZSBpbiBhIGh5cG90b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgZmxvd3MgaW50byB0aGUgY2VsbHMuIFN0dWR5IHRoZSBkaWFncmFtIGJlbG93IHRvIHNlZSB3aGF0IGNvbmRpdGlvbnMgd2lsbCBjYXVzZSBhbmltYWwgY2VsbHMgdG8gc2hyaW5rIGFuZCBzaHJpdmVsLg==
Cg==[Qq]
[f]WWVzLiBXaGVuIGFuaW1hbCBjZWxscyBhcmUgaW4gYSBoeXBlcnRvbmljIGVudmlyb25tZW50LCB3YXRlciBmbG93cyBvdXQgb2YgdGhlIGNlbGxzLiBUaGlzIG1ha2VzIHRoZW0gc2hyaW5rIGFuZCBzaHJpdmVsLg==
Cg==[Qq]
[f]Tm8uIElmIGFuaW1hbMKgY2VsbHMgYXJlIGluIGFuIGlzb3RvbmljIGVudmlyb25tZW50LCB3YXRlciB3aWxsIGJlIGVudGVyaW5nIGFuZCBsZWF2aW5nIHRoZW0gYXQgdGhlIHNhbWUgcmF0ZS7CoFN0dWR5IHRoZSBkaWFncmFtIGJlbG93IHRvIHNlZSB3aGF0IGNvbmRpdGlvbnMgd2lsbCBjYXVzZSBhbmltYWwgY2VsbHMgdG8gc2hyaW5rIGFuZCBzaHJpdmVsLg==
Cg==Cg==[Qq]
[q]When a plant is in this environment, it wilts.
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIFdoZW4gcGxhbnQgY2VsbHMgYXJlIGluIGEgaHlwb3RvbmljIGVudmlyb25tZW50LCB3YXRlciBmbG93cyBpbnRvIHRoZSBjZWxscy4gVGhpcyBleHBhbmRzIHRoZSBjZW50cmFsIHZhY3VvbGUgYW5kIHB1c2hlcyB0aGUgbWVtYnJhbmUgYWdhaW5zdCB0aGUgd2FsbC4gVGhhdCBtYWtlcyB0aGUgY2VsbHMgKGFuZCB0aGUgZW50aXJlIHBsYW50KSBzb2xpZCBhbmQgZmlybS4=
Cg==[Qq]
[f]WWVzLiBXaGVuIHBsYW50IGNlbGxzIGFyZSBpbiBhIGh5cGVydG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIGZsb3dzIG91dCBvZiB0aGUgY2VsbHMuIFRoaXMgY29udHJhY3RzIHRoZSBjZW50cmFsIHZhY3VvbGUgYW5kIHBlZWxzIHRoZSBtZW1icmFuZSBhd2F5IGZyb20gdGhlIHdhbGwuIFRoYXQgbWFrZXMgdGhlIGNlbGxzIChhbmQgdGhlIGVudGlyZSBwbGFudCkgZHJvb3B5IGFuZCB3aWx0ZWQu
Cg==[Qq]
[f]Tm8uIElmIHBsYW50IGNlbGxzIGFyZSBpbiBhbiBpc290b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgd2lsbCBiZSBlbnRlcmluZyBhbmQgbGVhdmluZyB0aGVtIGF0IHRoZSBzYW1lIHJhdGUuwqBUaGUgY2VsbHMgd2lsbCBiZSBpbiByZWFzb25hYmx5IGdvb2Qgc2hhcGUuIFdoYXQgb3Ntb3RpYyBjb25kaXRpb24gd291bGQgY2F1c2Ugd2F0ZXIgdG8gbGVhdmUgdGhlIGNlbGxzLCBjYXVzaW5nIHRoZSBwbGFudCB0byB3aWx0Pw==
Cg==Cg==[Qq]
[q]When animal cells are in this environment, they’ll expand, then burst.
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiBXaGVuIGFuaW1hbCBjZWxscyBhcmUgaW4gYSBoeXBvdG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIGZsb3dzIGludG8gdGhlIGNlbGxzLsKgVGhpcyBjYXVzZXMgdGhlbSB0byBleHBhbmQsIGFuZCBldmVudHVhbGx5IGJ1cnN0Lg==
Cg==[Qq]
[f]Tm8uIFdoZW4gYW5pbWFsIGNlbGxzIGFyZSBpbiBhIGh5cGVydG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIGZsb3dzIG91dCBvZiB0aGUgY2VsbHMuIFRoaXMgbWFrZXMgdGhlbSBzaHJpbmsgYW5kIHNocml2ZWwuIFdoYXQgb3Ntb3RpYyBjb25kaXRpb24gY2F1c2VzIHRoZW0gdG8gZXhwYW5kIGFuZCBidXJzdD8=
Cg==[Qq]
[f]Tm8uIElmIGFuaW1hbMKgY2VsbHMgYXJlIGluIGFuIGlzb3RvbmljIGVudmlyb25tZW50LCB3YXRlciB3aWxsIGJlIGVudGVyaW5nIGFuZCBsZWF2aW5nIHRoZW0gYXQgdGhlIHNhbWUgcmF0ZS4gVGhlaXIgc2l6ZSByZW1haW5zIHRoZSBzYW1lLiBTdHVkeSB0aGUgZGlhZ3JhbSBiZWxvdyB0byBzZWUgd2hhdCBjb25kaXRpb25zIHdpbGwgY2F1c2UgYW5pbWFsIGNlbGxzIHRvIGV4cGFuZCBhbmQgYnVyc3Q/
Cg==Cg==[Qq]
[q]If you want to keep animal tissue or cells alive outside the body, you have to keep them in what kind of osmotic environment?
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG 9uaWM=[Qq]
[f]Tm8uIFdoZW4gYW5pbWFsIGNlbGxzIGFyZSBpbiBhIGh5cG90b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgZmxvd3MgaW50byB0aGUgY2VsbHMuwqBUaGlzIGNhdXNlcyB0aGVtIHRvIGV4cGFuZCwgYW5kIGV2ZW50dWFsbHkgYnVyc3QuIFdoYXQgY29uZGl0aW9uIGtlZXBzIHRoZW0gdGhlIHNhbWUgc2l6ZSAod2hpY2ggaXMgdGhlIGhlYWx0aGllc3QgY29uZGl0aW9uIGZvciB0aGUgY2VsbHMpPw==
Cg==[Qq]
[f]Tm8uIFdoZW4gYW5pbWFsIGNlbGxzIGFyZSBpbiBhIGh5cGVydG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIGZsb3dzIG91dCBvZiB0aGUgY2VsbHMuIFRoaXMgbWFrZXMgdGhlbSBzaHJpbmsgYW5kIHNocml2ZWwuIFdoYXQgY29uZGl0aW9uIGtlZXBzIHRoZW0gdGhlIHNhbWUgc2l6ZSAod2hpY2ggaXMgdGhlIGhlYWx0aGllc3QgY29uZGl0aW9uIGZvciB0aGUgY2VsbHMpPw==
Cg==[Qq]
[f]WWVzLiBJZiBhbmltYWzCoGNlbGxzIGFyZSBpbiBhbiBpc290b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgd2lsbCBiZSBlbnRlcmluZyBhbmQgbGVhdmluZyB0aGVtIGF0IHRoZSBzYW1lIHJhdGUuIFRoZWlyIHNpemUgcmVtYWlucyB0aGUgc2FtZSwgd2hpY2ggaXMgdGhlIGhlYWx0aGllc3QgY29uZGl0aW9uIGZvciB0aGUgY2VsbHMu
Cg==[Qq]
[q]The cell on the right can be described as being [hangman]. The force of water moving into the cell is called [hangman] pressure.
[c]dHVyZ2lk[Qq]
[c]dHVyZ29y[Qq]
[x]
[restart]
[/qwiz]
11. Water Potential
The flow of water from hypotonic regions to hypertonic regions is one instance of a more general concept: water potential. Water potential includes osmotic pressure, but adds factors such as physical pressure, gravity, and surface tension to explain the movement of water.
When you see the term water potential, you should think of water potential energy. That’s because water potential is about water’s tendency to move from one area, where it has higher water potential, to an adjacent area, where the water potential is lower. This parallels how a rock on the top of a hill has high potential energy. A tiny push to overcome inertia will allow that rock to roll down the hill to where it has less potential energy. Similarly, water will spontaneously move from areas of high water potential to areas of low water potential.
While water potential has many dimensions, AP Bio students need to focus only on two:
- Osmolarity: adding solute to a solution (increasing its osmolarity) decreases its water potential. This entire tutorial has been devoted to helping you master how water moves, by osmosis, from hypotonic to hypertonic, and we’ll be applying that below.
- Pressure: Increasing the pressure on a body of water increases water potential. Think about what happens when you wring out a sponge: increased pressure in the sponge causes the water to leave the sponge.
Water potential is represented by the Greek letter Ψ, which is pronounced like “sigh.” Ψ is measured in units called megapascals (MPa), and it can be modeled by this equation:
Ψtotal = Ψs + Ψp
water potential = solute potential + pressure potential.
Let’s see this in action. Remember that water always flows from areas of higher water potential to areas with lower water potential.
[qwiz random=”false” style=”min-height: 350px !important;” qrecord_id=”sciencemusicvideosMeister1961-Water Potential (2.0)”]
[h]Water Potential Interactive Reading
[i]
The diagram at left shows a device called a U-tube. Imagine two curved test tubes that can be snapped together to create a U-shaped tube. At the point where the two connect, a selectively permeable membrane has been added that separates the solutions on the right and left sides.
[q multiple_choice=”true”] In the scenario illustrated below, the membrane is permeable to water, but not to the solute (sucrose). If you pour sugar into the left side of U-tube 2, what will happen?
[c]IFdhdGVyIHdpbGwgcmlzZSBvbiB0aGUgcmlnaHQgc2lkZSBvZiB0dWJlIDIgYmVjYXVzZSB0aGUgd2F0ZXIgcG90ZW50aWFsIGlzIGdyZWF0ZXIgb24gdGhlIHNpZGUgd2l0aCBwdXJlIHdhdGVyLg==[Qq]
[f]IE5vLiBBZGRpbmcgc29sdXRlIA==ZGVjcmVhc2VzIHdhdGVyIHBvdGVudGlhbC4gU2luY2Ugd2F0ZXIgYWx3YXlzIGZsb3dzIGZyb20gaGlnaGVyIHdhdGVyIHBvdGVudGlhbCB0byBsb3dlciB3YXRlciBwb3RlbnRpYWwsIHdhdGVyIHdpbGwgZmxvdyBmcm9tIHRoZSByaWdodCBzaWRlIG9mIHR1YmUgMiBpbnRvIGl0cyBsZWZ0IHNpZGUuIENsaWNrICYjODIyMDtOZXh0IFF1ZXN0aW9uJiM4MjIxOyB0byBzZWUgYW4gZXhwbGFuYXRvcnkgZGlhZ3JhbS4=[Qq]
[c]IFdhdGVyIHdpbGwgcmlzZSBvbiB0aGUgbGVmdCBzaWRlIG9mIHR1YmUgMiBiZWNhdXNlIHRoZS B3YXRlciBwb3RlbnRpYWwgaXMgbG93ZXIgb24gdGhlIHNpZGUgd2l0aCBhZGRlZCBzb2x1dGUu[Qq]
[f]IEV4Y2VsbGVudC4gV2F0ZXIgYWx3YXlzIGZsb3dzIGZyb20gaGlnaGVyIHRvIGxvd2VyIHBvdGVudGlhbC4gQWRkaW5nIHN1Y3Jvc2UgZGVjcmVhc2VzIHRoZSB3YXRlciBwb3RlbnRpYWwgb24gdGhlIGxlZnQgc2lkZSwgc28gd2F0ZXIgZmxvd3MgZnJvbSB0aGUgcmlnaHQgc2lkZSAod2l0aCBoaWdoZXIgd2F0ZXIgcG90ZW50aWFsKSB0byB0aGUgbGVmdCBzaWRlICh3aXRoIGxvd2VyIHdhdGVyIHBvdGVudGlhbCkuIENsaWNrICYjODIyMDtOZXh0IFF1ZXN0aW9uJiM4MjIxOyB0byBzZWUgYW4gZXhwbGFuYXRvcnkgZGlhZ3JhbS4=[Qq]
[c]IFRoZSB3YXRlciBsZXZlbCB3aWxsIHJpc2Ugb24gdGhlIGxlZnQgc2lkZSBiZWNhdXNlIHRoZSBhZGRlZCBzdWdhciBpbmNyZWFzZXMgdGhlIHZvbHVtZSBvbiB0aGF0IHNpZGUu[Qq]
[f]IE5vLiBUaGF0JiM4MjE3O3MgdHJ1ZSwgYnV0IGl0JiM4MjE3O3MgYnkgZmFyIG5vdCB0aGUgbW9zdCBpbXBvcnRhbnQgZmFjdG9yLiBUaGUgdGhpbmcgdG8gdGhpbmsgYWJvdXQgaGVyZSBpcyB3YXRlciBwb3RlbnRpYWwuIEFkZGluZyBzb2x1dGUgZGVjcmVhc2VzIHdhdGVyIHBvdGVudGlhbC4gU2luY2Ugd2F0ZXIgYWx3YXlzIGZsb3dzIGZyb20gaGlnaGVyIHdhdGVyIHBvdGVudGlhbCB0byBsb3dlciB3YXRlciBwb3RlbnRpYWwsIHdhdGVyIHdpbGwgZmxvdyBmcm9tIHRoZSByaWdodCBzaWRlIG9mIHR1YmUgMiBpbnRvIGl0cyBsZWZ0IHNpZGUuIENsaWNrICYjODIyMDtOZXh0IFF1ZXN0aW9uJiM4MjIxOyB0byBzZWUgYW4gZXhwbGFuYXRvcnkgZGlhZ3JhbS4=[Qq]
[q] Tube 3 shows what happens if you add solute to the left side of the U-tube. In terms of water potential, what happened is that the water potential on the right side of the tube is now [hangman] than the water potential on the left side of the tube. That’s because adding solute [hangman] water potential.
[c]IGhpZ2hlcg==[Qq]
[f]IEdvb2Qh[Qq]
[c]IGxvd2Vycw==[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[q] You should note that while the concept of water potential might be new, the scenario below can be explained just in terms of osmosis. Osmosis is the [hangman] of water. Adding solute made the left side [hangman] to the right side, and water always flows from [hangman] to hypertonic.
[c]IGRpZmZ1c2lvbg==[Qq]
[f]IEdvb2Qh[Qq]
[c]IGh5cGVydG9uaWM=[Qq]
[f]IEdyZWF0IQ==[Qq]
[c]IGh5cG90b25pYw==[Qq]
[f]IEdvb2Qh[Qq]
[q labels = “top”]Quantifying what happens when you add solute to one side of a U-tube is pretty straightforward. The pure water on the right side is not under pressure, and it has no dissolved solutes. Therefore its water potential will be 0 MPa. Start by dragging its water potential next to its Ψ symbol. There’s also no pressure on the solution on the left side, so you can drag the correct number to the Ψp (pressure potential) symbol on the left. Adding solute has lowered the solute potential of the left side, so drag the appropriate number to the Ψs symbol on the left, and then total everything up.
[l]0 MPa
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]-0.23 MPa
[fx] No, that’s not correct. Please try again.
[f*] Good!
[q]Note that while water potential caused water to flow from the right side to the left, that process won’t continue indefinitely. At a certain point, another force will counteract the force of solution potential: that’s a force that would pull the solution on the left side down. It’s not shown, and we haven’t discussed it, but you can probably guess what it is. It’s a fundamental force that pulls on things, also known as [hangman].
[c]Z3Jhdml0eQ==[Qq]
[q]Pressure is another component of water potential. Imagine that you took a plunger and used it to press down on the left side of the U-tube, as shown in “2.” Predict what will happen, and why.
[c]V2F0ZXIgd2lsbCByaXNlIG9uIHRoZSByaWdodCBzaWRlIG9mIHRoZSB0dWJlICh0aGUgc2lkZSB3aXRob3V0IHRoZSBwbHVuZ2VyKSBiZWNhdXNlIGl0IHdpbGzCoCBoYXZlIGEgaGlnaGVyIHByZXNzdXJlIHBvdGVudGlhbCB0aGFuIHRoZSBzaWRlIHdpdGggdGhlIHBsdW5nZXI=[Qq]
[f]Tm8uIFRoZSBwbHVuZ2VyIGlzIHB1c2hpbmcgZG93biBvbiB0aGUgbGVmdCBzaWRlLiBUaGF0JiM4MjE3O3Mgd2hlcmUgdGhlIHByZXNzdXJlIGlzIGdvaW5nIHRvIGJlLiBUaGF0IHdpbGwgaW5jcmVhc2UgdGhlIHdhdGVyIHBvdGVudGlhbCBvbiB0aGUgbGVmdCBzaWRlLiBJZiB3YXRlciBhbHdheXMgZmxvd3MgZnJvbSBoaWdoZXIgdG8gbG93ZXIgd2F0ZXIgcG90ZW50aWFsLCB0aGVuIGhvdyB3aWxsIHRoZSB3YXRlciBmbG93IGluIHJlc3BvbnNlPyBDbGljayAmIzgyMjA7TmV4dCBRdWVzdGlvbiYjODIyMTsgdG8gc2VlIGFuIGV4cGxhbmF0b3J5IGRpYWdyYW0u[Qq]
[c]VGhlIHdhdGVyIG9uIGJvdGggc2lkZXMgd2lsbCBzdGF5IGF0IHRoZSBzYW1lIGxldmVsIGJlY2F1c2UgdGhlIGZvcmNlIG9mIHRoZSBwbHVuZ2VyIHdpbGwgYmUgb2Zmc2V0IGJ5IHRoZSBmb3JjZSBvZiBncmF2aXR5Lg==[Qq]
[f]Tm8uIEdyYXZpdHkgY2FuIGJlIGEgY29tcG9uZW50IG9mIHdhdGVyIHBvdGVudGlhbC4gQnV0IHlvdSYjODIxNzt2ZSBqdXN0IHNlZW4gaG93IGFkZGluZyBzb2x1dGUgdG8gb25lIHNpZGUgY2F1c2VkIHRoZSBmbHVpZCB0byByaXNlIG9uIHRoYXQgc2lkZSwganVzdCBiZWNhdXNlIG9mIHRoZSBmb3JjZSBvZiBvc21vc2lzLCB3aGljaCBtdXN0IGhhdmUgcHVzaGVkIHdpdGggYSBmb3JjZSB0aGFuIGV4Y2VlZGVkIGdyYXZpdHkmIzgyMTc7cyBkb3dud2FyZCBwdWxsLiBTbywgaWdub3JlIGdyYXZpdHkgZm9yIG5vdy4gSnVzdCB0aGluayB0aGF0IGlmIHRoZSBwbHVuZ2VyIGlzIG9uIHRoZSBsZWZ0IHNpZGUsIHRoZW4gdGhhdCYjODIxNztzIHdoZXJlIHRoZSBwcmVzc3VyZSBpcyBnb2luZyB0byBiZS4gVGhhdCB3aWxsIGluY3JlYXNlIHRoZSB3YXRlciBwb3RlbnRpYWwgb24gdGhlIGxlZnQgc2lkZS4gSWYgd2F0ZXIgYWx3YXlzIGZsb3dzIGZyb20gaGlnaGVyIHRvIGxvd2VyIHdhdGVyIHBvdGVudGlhbCwgdGhlbiBob3cgd2lsbCB0aGUgd2F0ZXIgZmxvdyBpbiByZXNwb25zZT8gQ2xpY2sgJiM4MjIwO05leHQgUXVlc3Rpb24mIzgyMjE7IHRvIHNlZSBhbiBleHBsYW5hdG9yeSBkaWFncmFtLg==[Qq]
[c]VGhlIGRvd253YXJkIGZvcmNlIG9mIHRoZSBwbHVuZ2VyIHdpbGwgaW5jcmVhc2UgdGhlIHdhdGVyIHBvdGVudGlhbCBvbiB0aGUgbGVmdCBzaWRlLiBCZWNhdXNlIHdhdGVyIGFsd2F5cyBmbG 93cyBmcm9tIGhpZ2hlciB0byBsb3dlciB3YXRlciBwb3RlbnRpYWwsIHRoZSB3YXRlciB3aWxsIGZsb3cgZnJvbSBsZWZ0IHRvIHJpZ2h0LCBhbmQgcmlzZSBvbiB0aGUgcmlnaHQgc2lkZS4=[Qq]
[f]TmljZSBqb2IhIFByZXNzdXJlIGluY3JlYXNlcyB3YXRlciBwb3RlbnRpYWwuIFRoYXQgd2lsbCBwdXNoIHRoZSB3YXRlciBhd2F5IGZyb20gdGhlIGxlZnQgc2lkZSBhbmQgcHVzaCBpdCB0b3dhcmQgdGhlIHJpZ2h0IHNpZGUuIENsaWNrICYjODIyMDtOZXh0IFF1ZXN0aW9uJiM4MjIxOyB0byBzZWUgYW4gZXhwbGFuYXRvcnkgZGlhZ3JhbS4=[Qq]
[q]The downward force of the plunger increases the [hangman] potential on the left side. Because the formula for water potential, is Ψtotal = Ψs + Ψp, we know that increasing Ψp will increase the overall [hangman] potential. Because water always flows from [hangman] to [hangman] water potential, it moves from the left side to the right side.
[c]cHJlc3N1cmU=[Qq]
[c]d2F0ZXI=[Qq]
[c]aGlnaGVy[Qq]
[c]bG93ZXI=[Qq]
[q]Now that we understand the two components of water potential, we can make a few predictions about the consequences of water potential in living cells. If you place a flaccid cell in pure water (Ψ = 0.0 MPa, as shown below), what will happen to the cell? Note that in this scenario, the cytoplasm has a Ψs of -1.0 MPa.
[c]VGhlIGNlbGwgd2lsbCBsb3NlIHdhdGVyIGFzIHRoZSB3YXRlciBmbG93cyBmcm9tIHRoZSBjeXRvcGxhc20sIHdoZXJlIHRoZSB3YXRlciBwb3RlbnRpYWwgaXMgbG93ZXIsIHRvIHRoZSBiZWFrZXIsIHdoZXJlIHRoZSB3YXRlciBwb3RlbnRpYWwgaXMgaGlnaGVyLg==[Qq]
[f]Tm8uIExvb2sgYXQgdGhlIHZhbHVlIG9mIM6oIGluIHRoZSBiZWFrZXIuIEl0JiM4MjE3O3MgMC4wIE1QYS4gVGhhdCYjODIxNztzIGhpZ2hlciB0aGFuIHRoZSB2YWx1ZSBvZiDOqCBpbiB0aGUgY2VsbCwgd2hlcmUgaXQmIzgyMTc7cyAtMS4wIE1QYS4gQmVjYXVzZSB3YXRlciBhbHdheXMgZmxvd3MgZnJvbSBoaWdoZXIgdG8gbG93ZXIgd2F0ZXIgcG90ZW50aWFsLCBpdCB3aWxsIGZsb3cgaW50byB0aGUgY2VsbC4=[Qq]
[c]VGhlIGNlbGwgd2lsbCBnYWluIHdhdGVyIGFzIHRoZSB3YXRlciBmbG93cyBmcm9tIHRoZSBiZWFrZXIsIHdoZXJlIHRoZSB3YX RlciBwb3RlbnRpYWwgaXMgaGlnaGVyLCB0byB0aGUgY2VsbCwgd2hlcmUgdGhlIHdhdGVyIHBvdGVudGlhbCBpcyBsb3dlci4=[Qq]
[f]TmljZSBqb2IuIEJlY2F1c2Ugd2F0ZXIgYWx3YXlzIGZsb3dzIGZyb20gaGlnaGVyIHRvIGxvd2VyIHdhdGVyIHBvdGVudGlhbCwgd2F0ZXIgd2lsbCBmbG93IGludG8gdGhlIGNlbGwu[Qq]
[q]Beaker number 2 shows what will happen to beaker number 1 over time. Because the water in the beaker has a water potential of [hangman] MPa, water will flow from the beaker, where the water potential is [hangman], into the cell. What’s lowering the cell’s water potential is the [hangman] in the cytoplasm. In beaker 2, the [hangman] potential, which has risen to 1.0 MPa, is counteracting the -1.0 MPa [hangman] potential, so the cell’s shape has changed. The name for the force of the cytoplasm pushing out against the cell wall is [hangman] pressure.
[c]MC4w[Qq]
[c]aGlnaGVy[Qq]
[c]c29sdXRl[Qq]
[c]cHJlc3N1cmU=[Qq]
[c]c29sdXRl[Qq]
[c]dHVyZ29y[Qq]
[x]
[/qwiz]
12. Osmosis Rap
13. Osmosis Karaoke
What’s next?
With this tutorial, you’ve made it through all of the learning objectives in AP Biology Unit 2. Go to the Unit 2 Flashcards and Cumulative Quizzes to consolidate your understanding of everything you’ve learned.
