Cellular Respiration Student Learning Guide
1. ATP is at the center of biology
If there was a prize for the most important biological molecule, you might want to consider nominating ATP, which stands for adenosine triphosphate.
.jpg)
ATP is a nucleotide monomer. It’s composed of 3 subparts.
- Part 1 is the five-carbon sugar ribose.
- Part 2 is the nitrogenous base adenine, which is one of the letters of the genetic code.
- Part 3 consists of three phosphate groups (which is where the “triphosphate” part of ATP’s name comes from).
When you think of nucleotides, you might think of “information,” since that’s the role that nucleic acids like DNA and RNA play in living systems. Nucleic acids are nucleotide polymers, and ATP is one of the nucleotide monomers of RNA. In some viruses (like SARS-CoV-2 and HIV) RNA is the genetic material. In cells, RNA’s key role is transmitting genetic information from DNA and translating that information into protein.
ATP is also life’s energy carrier. In the shortest timespan, ATP is how living things store energy, and then release energy to perform the work of life. Want an example? Wiggle your finger. That movement required muscle contraction. The energy for that contraction was directly powered by ATP. Even thinking about moving your finger was powered by ATP (though less directly. If you’re interested, follow this link to a tutorial about nerve impulses to learn how). :
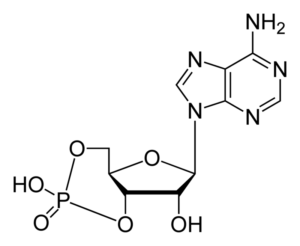
But there’s more. When cells need to respond to messages received from outside the cell, they do this through a second messenger system that’s based on a modified form of ATP called cyclic AMP. The “M” in “AMP” stands for “mono”, and notice the single phosphate group that’s connected to ribose.
So, information, energy transfer, and cellular response. As I said above, that makes ATP is a good candidate for the most important molecule in biology. Below, we’ll see how ATP works as an energy currency. But first, let’s check to see how much you retained from what’s above.
[qwiz random=”true” style “width = 540px” qrecord_id=”sciencemusicvideosMeister1961-ATP Quiz 1 (M10)”]
[h]ATP is at the center of biology: Quiz
[i]
[q labels = “top”]Let’s try some labeling.
[l]adenine (nitrogenous base)
[fx] No. Please try again.
[f*] Great!
[l]three phosphate groups
[fx] No. Please try again.
[f*] Excellent!
[l]ribose sugar
[fx] No. Please try again.
[f*] Great!
[q]In a nucleotide polymer, this part is one of the four letters
[textentry single_char=”true”]
[c]Mg ==[Qq]
[f]WWVzLiAmIzgyMjA7MiYjODIyMTsgaXPCoGFkZW5pbmUsIG9uZSBvZiB0aGUgZm91ciBsZXR0ZXJzIG9mIHRoZSBnZW5ldGljIGFscGhhYmV0IChBLCBULCBDLCBHIGluIEROQTsgQSwgVSwgQywgRyBpbiBSTkEp[Qq]
[c]Kg==[Qq]
[f]Tm8uIExvb2sgZm9yIHRoZSBuaXRyb2dlbm91cyBiYXNlLiBKdXN0IHRoaW5rIGFib3V0IHdoaWNoIHBhcnQgY291bGQgYmUgZGVzY3JpYmVkIGFzICYjODIyMDtuaXRyb2dlbm91cy4mIzgyMjE7
Cg==[Qq]
[q]Which part is the sugar ribose?
[textentry single_char=”true”]
[c]MQ ==[Qq]
[f]WWVzLiAmIzgyMjA7MSYjODIyMTsgaXPCoHRoZSBzdWdhciByaWJvc2Uu[Qq]
[c]Kg==[Qq]
[f]Tm8uIFN1Z2FycyB0eXBpY2FsbHkgaGF2ZSBhIGhleGFnb25hbCAoc2l4LXNpZGVkKSBvciBwZW50YWdvbmFsICg1IHNpZGVkKSBzdHJ1Y3R1cmUuIFdoaWNoIG9mIHRoZSBudW1iZXJlZCBwYXJ0cyBhYm92ZSBsb29rcyBsaWtlIGEgc3VnYXI/
Cg==[Qq]
[q]Which part is involved in energy transfer?
[textentry single_char=”true”]
[c]Mw ==[Qq]
[f]WWVzLiBUaGUgcGhvc3BoYXRlIGdyb3VwcyBhdCAmIzgyMjA7MyYjODIyMTsgYXJlIHdoZXJlIGFuZCBob3cgQVRQIHN0b3JlcyBhbmQgcmVsZWFzZXMgZW5lcmd5Lg==[Qq]
[c]Kg==[Qq]
[f]Tm8uIEl0JiM4MjE3O3MgdGhlIHBob3NwaGF0ZSBncm91cHMgdGhhdCBwbGF5IHRoaXMgcm9sZS4gV2hpY2ggaXMgdGhlIG9ubHkgcGFydCB0aGF0IGNvdWxkIGJlIGEgcGhvc3BoYXRlIGdyb3VwPw==
Cg==[Qq]
[q]ATP is a nucleotide __________
[hangman]
[c]bW9ub21lcg==[Qq]
[f]WWVzLiBBVFAgaXMgYSBudWNsZW90aWRlIA==bW9ub21lcg==Lg==
Cg==[Qq]
[q]Which molecule below is ATP?
| 1 | 2 | 3 |
[textentry single_char=”true”]
[c]Mw ==[Qq]
[f]WWVzLiBUaGUgdGhyZWUgcGhvc3BoYXRlIGdyb3VwcyBhdCAmIzgyMjA7MyYjODIyMTsgbWFrZSB0aGlzIG1vbGVjdWxlICYjODIyMDtBVFAsJiM4MjIxOyBvciA=YWRlbm9zaW5lIHRyaXBob3NwaGF0ZS4=[Qq]
[c]Kg==[Qq]
[f]Tm8uIENvdW50IHRoZSBudW1iZXIgb2YgcGhvc3BoYXRlIGdyb3VwcyBhdHRhY2hlZCB0byBlYWNoIG1vbGVjdWxlLiBXaGF0IGRvZXMgdGhlICYjODIyMDtUJiM4MjIxOyBpbiAmIzgyMjA7QVRQJiM4MjIxOyBzdGFuZCBmb3I/
Cg==[Qq]
[q]ATP (in a slightly modified form) also serves as one of the “letters” in which of the following polymers?
[c]RE5B[Qq]
[c]Uk 5B[Qq]
[c]cHJvdGVpbg==[Qq]
[c]cG9seXNhY2NoYXJpZGVz[Qq]
[f]Tm8sIGJ1dCB5b3UmIzgyMTc7cmUgdmVyeSBjbG9zZS4gQVRQIGlzIGEgbW9ub21lciBvZiBvbmUgb2YgdGhlIG51Y2xlb3RpZGUgcG9seW1lcnMsIGJ1dCBub3QgRE5BICh0aGUgb25lIHlvdSYjODIxNztyZSBwb3NzaWJseSBtb3N0IGZhbWlsaWFyIHdpdGgpLiBXaGF0JiM4MjE3O3MgdGhlIA==b3RoZXI=IG51Y2xlb3RpZGUgcG9seW1lci4=[Qq]
[f]Q29ycmVjdCEgQVRQIChpbiBhIHNsaWdodGx5IG1vZGlmaWVkIGZvcm0pIGFsc28gc2VydmVzIGFzIG9uZSBvZiB0aGUgbGV0dGVycyBvZiBSTkEu[Qq]
[f]Tm8uIFRoZSBsZXR0ZXJzIHRoYXQgbWFrZSB1cCBwcm90ZWluIGFyZSBhbWlubyBhY2lkcy4gSXQmIzgyMTc7cyBvbmUgb2YgdGhlIHR3byBpbmZvcm1hdGlvbmFsIG51Y2xlb3RpZGVzIG9uIHRoZSBsaXN0Lg==[Qq]
[f]Tm8uIFBvbHlzYWNjaGFyaWRlcyBhcmUgY2FyYm9oeWRyYXRlIHBvbHltZXJzLiBUaGV5JiM4MjE3O3JlIG1hZGUgb2YgbW9ub21lcnMsIGJ1dCB0aGUgJiM4MjIwO2xldHRlciYjODIyMTsgbm90aW9uIGRvZXNuJiM4MjE3O3QgYXBwbHkgdG8gdGhlbS4gTWFrZSBhbm90aGVyIGNob2ljZSB3aGVuIHlvdSBzZWUgdGhpcyBxdWVzdGlvbiBhZ2Fpbi4=[Qq]
[x]
[restart]
[/qwiz]
2. Life, Entropy, Energy, and ATP
In the introduction, ATP was described as life’s energy carrier. What is energy? What does it mean to carry energy? And why are living things so energy-dependent?
Organisms — anything from an E. coli bacterium to a redwood tree to a human being like yourself — are systems that are highly complex and highly organized. Consider DNA. Its double-helical structure is the height of organization. Its sequence of bases is organized to store and transmit genetic information. Move up from molecules to cells, tissues, organs, etc, and you’ll this organization and complexity over and over again
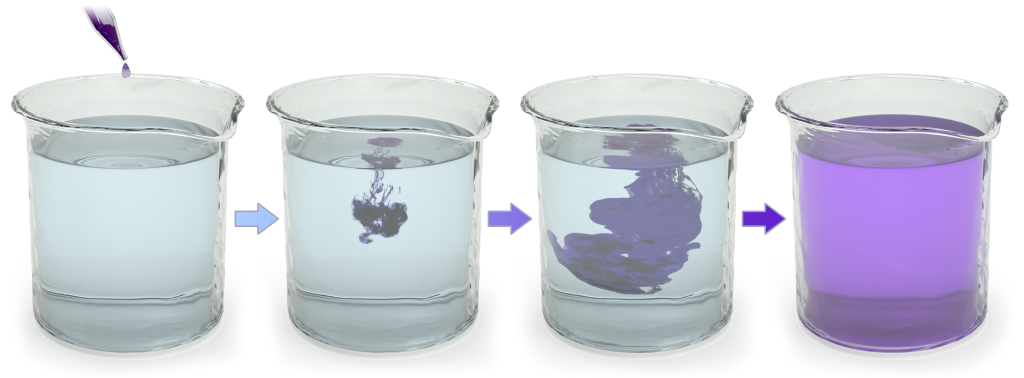
Complexity and order are inherently unstable conditions. That’s because of a process called entropy. Entropy is the tendency of any system to spontaneously become more disorganized. Add a drop of ink to a glass of water. Over time, that ink spreads out, becoming a diffuse mixture of ink spread out in the water. That’s entropy.
Entropy is why complex systems wear down over time. Think of any machine (a bicycle, a seesaw, etc.). As you use it (unless you do the work of maintaining it and repairing it) it’ll eventually wear down. That’s entropy. Think of your bedroom. Unless you put energy into maintaining its order, your use of the room will cause it to become more disorganized over time. That’s also entropy.
Entropy is a basic feature of the universe. It’s been called “time’s arrow.” But in this universe, which is constantly moving from more organized to less organized, living things are capable of creating highly organized structures, maintaining these structures, and reproducing these structures. How?
Fighting against entropy to create, maintain, and reproduce life’s organization requires energy. Energy can be defined as the ability to do work. Living things require a constant input of energy to stay alive. That doesn’t mean that you need to be eating every second: organisms can store energy in the chemical bonds of carbohydrates, lipids, and other molecules. But over time, in any organism, energy input needs to exceed energy use. If it doesn’t, the complex organization that underlies life won’t be maintained. Death is the result.
ATP is how living things, at the cellular level, carry out the work of life. ATP’s key role is to couple energy input to work. Consider, for example, the reaction below.
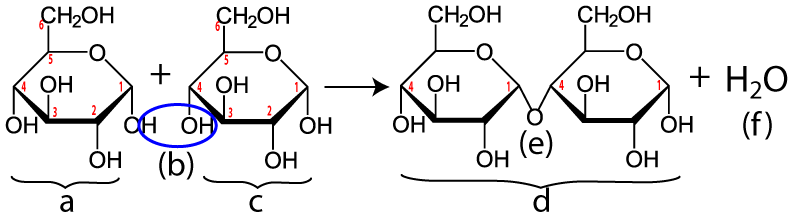
Two glucose molecules (letters “a” and “c” above) are being combined to form the disaccharide maltose (at “d”). That’s a decrease in entropy: one maltose molecule is more organized than two glucose molecules. Reducing entropy takes work.
To power that work, cells will convert ATP into its lower energy counterpart, ADP and phosphate.
![]()
Since cells are constantly using up ATP to power work, they also need to replenish their ATP supply. Cells do that by converting the chemical energy in food (typically glucose, but also other molecules) into ATP. The details of that process — converting food energy into ATP — are the subject of this and the next tutorials.
3. Releasing chemical energy through combustion

Before we go into the details of how ATP stores and releases energy, let’s take a look at how chemical energy gets released in some systems that aren’t alive, where what happens is much simpler.
One way to release stored chemical energy is combustion. Combustion is what happens when wood burns:
WOOD + oxygen –> energy (heat and light) + carbon dioxide + water
What this means is that the molecules in the wood combine with oxygen in the air. This releases heat and light energy, along with two waste products: carbon dioxide (CO2) and water (H2O). Carbon dioxide is a colorless and odorless gas. The water released is in the form of water vapor, or steam.

Burning gasoline in a car is also combustion.
Gasoline + oxygen –> energy (heat) + carbon dioxide + water
The car’s engine transforms the heat into kinetic energy (motion). If you stand behind a car on a cold day, you can see the water released as steam goes into the air.
Take the quiz below to check your understanding of combustion, energy, entropy, and ATP
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Combustion, Checking Understanding”]
[h]Checking Understanding: Combustion, Energy, Entropy, and ATP
[q labels = “top”]Note: not every term below has to be used to answer this question.
_______ + oxygen –> energy (heat) + ________ + water
[l]energy
[fx] No. Please try again.
[f*] Great!
[l]CO2
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]fuel
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[q labels = “top”]
FUEL + _________ –> energy + carbon dioxide + _______
[l]oxygen
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]water
[fx] No, that’s not correct. Please try again.
[f*] Good!
[q]The complexity and order found in living things
[c]YS4gcmVxdWlyZXMgZW5lcmd5IHRvIG1haW50YWlu[Qq]
[c]Yi4gZ29lcyBhZ2FpbnN0IHRoZSBwcm9jZXNzIG9mIGVudHJvcHk=[Qq]
[c]Yy4gaXMgaGlnaGx5IHVuc3RhYmxl[Qq]
[c]ZC4gYWxsIG9mIH RoZSBhYm92ZQ==[Qq]
[q]The tendency of any system to move from organized to disorganized is known as [hangman].
[c]ZW50cm9weQ==[Qq]
[q]Fighting against entropy requires an input of [hangman].
[c]ZW5lcmd5[Qq]
[q labels = “top”]Over time, in any organism, energy _____________ needs to exceed energy _________. If not, the result is ________.
[l]death
[fx] No. Please try again.
[f*] Correct!
[l]input
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]loss
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]To power the work of staying alive, cells convert _____________ into its lower energy form: _______ and ________.
[l]ADP
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]ATP
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]phosphate
[fx] No. Please try again.
[f*] Excellent!
[/qwiz]
4. In cells, food energy gets transformed into ATP
In a car, the engine combusts a fuel like gasoline, transforming the chemical energy in the fuel into heat. Then the heat is transformed into kinetic energy: energy of motion.
In living cells, things are more complex. In animals like us, the fuel comes from food, and to make the energy in food available for cellular work, cells first have to transfer the chemical bond energy in the molecules in the food we eat into the chemical bond energy in one of ATP’s chemical bonds: the bond indicated by “A” below, which holds ATPs third phosphate group to its second phosphate group.
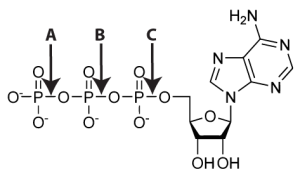
Why? Note that each phosphate group consists of one phosphorus atom surrounded by oxygen atoms. As you can see in the diagram, the oxygen shown on the bottom of each phosphate has a negative charge. Negative charges repel one another. In other words, each of these phosphate groups is simultaneously bonded to the others but also pushing away.
Almost all of the energy transfer in living things is catalyzed by enzymes (which you can learn more about in my enzyme tutorial and song). A typical enzymatic move to get some work done for the cell involves breaking off the last phosphate in ATP and attaching that phosphate to another molecule. Breaking the bond that holds the last phosphate onto ATP requires only a small amount of energy. Attaching the phosphate onto another molecule, however, releases enough energy to power the overall reaction. And that’s how the work of life takes place. It’s how you move your muscles. It’s how your nerve cells set themselves up to send impulses, which allows you to do the thinking about ATP that you’re doing now.
5. The ATP/ADP cycle is how cells release and store energy
To repeat: when a cell needs to release a bit of energy to get some work done, it will, through the action of an enzyme, break off the last phosphate in ATP, and place that phosphate onto another molecule. This releases a small amount of energy and transforms ATP into its counterpart, ADP.
Here’s the structure of ATP and ADP, side by side. ADP stands for Adenosine di-phosphate, and as you can see below, it has two phosphate groups. Note that the last phosphate group in ATP or ADP can be shown either in an ionized (charged form) or an un-ionized (uncharged form). Below, ATP is shown in its uncharged form (with an -OH group on its last phosphate). ADP is shown in its charged form (note the oxygen with a minus sign). In textbooks and tests you can see either form, so get used to seeing both.
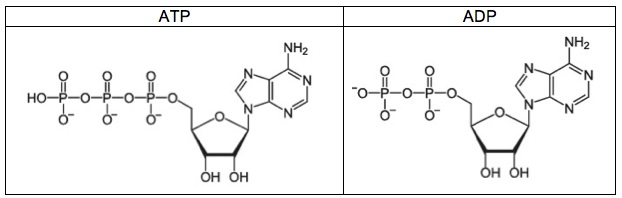
You can think of ADP as a rechargeable battery that has run out of electrical energy. ATP, with its three phosphates, is like a fully charged up battery, ready to power whatever it is that a cell needs to do.
ATP and ADP are linked in a cycle. See if by analyzing what you see below you can figure out the parts.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-ATP-ADP Cycle, Interactive Diagram”]
[h]Interactive Diagram: ATP/ADP Cycle
[q labels = “top”]
[l]ADP
[fx] No. Please try again.
[f*] Correct!
[l]ATP
[fx] No. Please try again.
[f*] Excellent!
[l]Energy from food
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]Energy released for work
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]Phosphate group
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[/qwiz]
Cells convert ATP into ADP and phosphate to do some work. For example, when you’re kicking a ball, the contraction of your muscles is powered by the conversion of trillions of ATPs into ADP and phosphate. And this is made possible by the food you eat, which powers the creation of ADP and P into ATP.
6. Life, Energy, and ATP: Checking Understanding
In the next unit, we’ll start to look at how cells make ATP in more detail. But for now, make sure you’ve got the big picture by taking the quiz below.
[qwiz random = “true” qrecord_id=”sciencemusicvideosMeister1961-Combustion, ATP, ADP (M10)”]
[h]Quiz: Combustion, ATP, ADP
[q topic= “ATP”]When you strike a match, the chemical energy in the match is released in the form of
[c]IEFEUA==[Qq]
[c]IEhlYXQgYW 5kIGxpZ2h0[Qq]
[c]IGNhcmJvbiBkaW94aWRl[Qq]
[f]IE5vLiBTdHJpa2luZyBhIG1hdGNoIGlzIGFuIGV4YW1wbGUgb2YgY29tYnVzdGlvbi4gQURQIGlzIHByb2R1Y2VkIHdoZW4gQVRQIGlzIHVzZWQgYnkgY2VsbHMuIFdoYXQgZG8geW91IHNlZSBhbmQgZmVlbCBmcm9tIGEgbGlnaHRlZCBtYXRjaD8=[Qq]
[f]IENvcnJlY3QuIFRoaXMgaXMgYW4gZXhhbXBsZSBvZiBjb21idXN0aW9uLCBhbmQgdGhlIGVuZXJneSBpcyByZWxlYXNlZCBhcyBoZWF0IGFuZCBsaWdodC4=[Qq]
[f]IE5vLiBDYXJib24gZGlveGlkZSBpcyBvbmUgb2YgdGhlIHdhc3RlIHByb2R1Y3RzIG9mIGEgY29tYnVzdGlvbiByZWFjdGlvbiwgYnV0IGl04oCZcyBub3QgZW5lcmd5ICYjODIxMTsgaXTigJlzIGFuIGV4aGF1c3QgcHJvZHVjdC4gV2hhdCBkbyB5b3Ugc2VlIGFuZCBmZWVsIGZyb20gYSBsaWdodGVkIG1hdGNoPw==
[q topic= “ATP”]In living things, the chemical energy in food is transformed into
[c]IHRoZSBjaGVtaWNhbC BlbmVyZ3kgaW4gQVRQ[Qq]
[c]IEhlYXQgYW5kIGxpZ2h0[Qq]
[c]IGNhcmJvbiBkaW94aWRl[Qq]
[f]IFllcy4gTGl2aW5nIHRoaW5ncyBoYXZlIHRvIHRyYW5zZmVyIHRoZSBlbmVyZ3kgaW4gZm9vZHMgKHN1Y2ggYXMgZ2x1Y29zZSkgaW50byB0aGUgZW5lcmd5IGluIEFUUC4=[Qq]
[f]IE5vLiBQcm9kdWN0aW9uIG9mIGhlYXQgYW5kIGxpZ2h0IGlzIHdoYXQgaGFwcGVucyB3aGVuIGEgZnVlbCBpcyBidXJuZWQuIEJ1dCB0aGF04oCZcyBub3Qgd2hhdCBoYXBwZW5zIGluIGxpdmluZyB0aGluZ3Mu[Qq]
[f]IE5vLiBDYXJib24gZGlveGlkZSBpcyBvbmUgb2YgdGhlIHdhc3RlIHByb2R1Y3RzIG9mIGNlbGx1bGFyIHJlc3BpcmF0aW9uLCB3aGljaCBpcyB0aGUgcHJvY2VzcyB0aGF0IGNlbGxzIHVzZSB0byBnZXQgZW5lcmd5IGZyb20gZm9vZC4gQnV0IGNhcmJvbiBkaW94aWRlIGlzIGEgd2FzdGUgcHJvZHVjdC4gTG9vayBmb3Igc29tZXRoaW5nIHRoYXQgY2VsbHMgY2FuIHVzZSB0byBwb3dlciB0aGVpciBsaWZlIHByb2Nlc3Nlcy4=
[q topic= “ATP”]In this diagram of ATP, the phosphate groups are shown at
[c]IDE=[Qq]
[c]IDI=[Qq]
[c]ID M=[Qq]
[f]IE5vLiBOdW1iZXIgMSBpcyB0aGUgc3VnYXIsIHJpYm9zZS4gVG8gZmluZCB0aGUgcGhvc3BoYXRlIGdyb3VwcywgbG9vayBmb3IgdGhlIGNoZW1pY2FsIHN5bWJvbCBmb3IgcGhvc3Bob3J1cywg4oCYUC7igJk=[Qq]
[f]IE5vLiBOdW1iZXIgMiBpcyB0aGUgbml0cm9nZW5vdXMgYmFzZSwgYWRlbmluZS4gVG8gZmluZCB0aGUgcGhvc3BoYXRlIGdyb3VwcywgbG9vayBmb3IgdGhlIGNoZW1pY2FsIHN5bWJvbCBmb3IgcGhvc3Bob3J1cywg4oCYUC7igJk=[Qq]
[f]IFllcy4gTnVtYmVyIDMgcmVmZXJzIHRvIHRoZSBwaG9zcGhhdGUgZ3JvdXBzLCB3aGljaCBpcyB3aGVyZSBBVFAgc3RvcmVzIGl0cyBlbmVyZ3ku
[q topic= “ATP”]In this diagram of ATP, the nitrogenous base adenine is found at
[c]IDE=[Qq]
[c]ID I=[Qq]
[c]IDM=[Qq]
[f]IE5vLiBOdW1iZXIgMSBpcyB0aGUgc3VnYXIsIHJpYm9zZS4gVG8gZmluZCB0aGUgbml0cm9nZW5vdXMgYmFzZSBhZGVuaW5lLCBsb29rIGZvciB0aGUgbml0cm9nZW4tY29udGFpbmluZyByaW5ncy4g4oCYTuKAmSBpcyB0aGUgc3ltYm9sIGZvciBuaXRyb2dlbi4=[Qq]
[f]IFllcyEgTnVtYmVyIDIgaXMgdGhlIG5pdHJvZ2Vub3VzIGJhc2UsIGFkZW5pbmUu[Qq]
[f]IE5vLiBOdW1iZXIgMyByZWZlcnMgdG8gdGhlIHBob3NwaGF0ZSBncm91cHMsIHdoaWNoIGlzIHdoZXJlIEFUUCBzdG9yZXMgaXRzIGVuZXJneS4gVG8gZmluZCB0aGUgbml0cm9nZW5vdXMgYmFzZSBhZGVuaW5lLCBsb29rIGZvciB0aGUgbml0cm9nZW4tY29udGFpbmluZyByaW5ncy4g4oCYTuKAmSBpcyB0aGUgc3ltYm9sIGZvciBuaXRyb2dlbi4=
[q topic= “ATP”]In this diagram of ATP, the sugar ribose is found at
[c]ID E=[Qq]
[c]IDI=[Qq]
[c]IDM=[Qq]
[f]IFllcy4gTnVtYmVyIDEgaXMgdGhlIHN1Z2FyLCByaWJvc2UsIHdoaWNoIHlvdSBjYW4gaWRlbnRpZnkgYnkgaXRzIHBlbnRhZ29uYWwgc2hhcGUu[Qq]
[f]IE5vLiBOdW1iZXIgMiBpcyB0aGUgbml0cm9nZW5vdXMgYmFzZSwgYWRlbmluZS4gWW91IGNhbiBpZGVudGlmeSB0aGUgc3VnYXIgcmlib3NlIGJ5IGl0cyBwZW50YWdvbmFsIHNoYXBlLg==[Qq]
[f]IE5vLiBOdW1iZXIgMyByZWZlcnMgdG8gdGhlIHBob3NwaGF0ZSBncm91cHMsIHdoaWNoIGlzIHdoZXJlIEFUUCBzdG9yZXMgaXRzIGVuZXJneS4gWW91IGNhbiBpZGVudGlmeSB0aGUgc3VnYXIgcmlib3NlIGJ5IGl0cyBwZW50YWdvbmFsIHNoYXBlLg==
[q topic= “ATP”]In this diagram of ATP, the part that contains chemical bonds that are broken down for energy is
[c]IDE=[Qq]
[c]IDI=[Qq]
[c]ID M=[Qq]
[f]IE5vLiBOdW1iZXIgMSBpcyB0aGUgc3VnYXIsIHJpYm9zZS4gSW4gQVRQLCBpdOKAmXMgdGhlIGxhc3QgcGhvc3BoYXRlIGdyb3VwIHRoYXQgZ2V0cyBicm9rZW4gb2ZmIHRoZSBtb2xlY3VsZSB0byByZWxlYXNlIGVuZXJneSBmb3IgY2VsbHVsYXIgd29yay4gV2hpY2ggbnVtYmVyIGlzIHBvaW50aW5nIHRvIHRoZSBwaG9zcGhhdGUgZ3JvdXBzPw==[Qq]
[f]IE5vLiBOdW1iZXIgMiBpcyB0aGUgbml0cm9nZW5vdXMgYmFzZSwgYWRlbmluZS4gSW4gQVRQLCBpdOKAmXMgdGhlIGxhc3QgcGhvc3BoYXRlIGdyb3VwIHRoYXQgZ2V0cyBicm9rZW4gb2ZmIHRoZSBtb2xlY3VsZSB0byByZWxlYXNlIGVuZXJneSBmb3IgY2VsbHVsYXIgd29yay4gV2hpY2ggbnVtYmVyIGlzIHBvaW50aW5nIHRvIHRoZSBwaG9zcGhhdGUgZ3JvdXBzPw==[Qq]
[f]IFllcy4gTnVtYmVyIDMgcmVmZXJzIHRvIHRoZSBwaG9zcGhhdGUgZ3JvdXBzLCB3aGljaCBpcyB3aGVyZSBBVFAgc3RvcmVzIGl0cyBlbmVyZ3kuIEJ5IGJyZWFraW5nIG9mZiB0aGUgbGFzdCBwaG9zcGhhdGUgZ3JvdXAsIGNlbGxzIHJlbGVhc2UgZW5lcmd5IGZvciBjZWxsdWxhciB3b3JrLg==
[q topic= “ATP”]When ATP is converted to ADP and Phosphate, which chemical bond is broken?
[c]IE E=[Qq]
[c]IEI=[Qq]
[c]IEM=[Qq]
[f]IFllcy4gQnJlYWtpbmcgdGhlIGJvbmQgYXQg4oCYQeKAmSBjb252ZXJ0cyBBVFAgdG8gQURQIGFuZCBwaG9zcGhhdGUsIHJlbGVhc2luZyBlbmVyZ3kgZm9yIGNlbGx1bGFyIHdvcmsu[Qq]
[f]IE5vLiBUbyBjb252ZXJ0IEFUUCB0byBBRFAgYW5kIHBob3NwaGF0ZSwgdGhlIG91dGVybW9zdCBwaG9zcGhhdGUgZ3JvdXAgaXMgYnJva2VuIG9mZi4gV2hpY2ggYm9uZCB3b3VsZCBoYXZlIHRvIGJyZWFrIHRvIHJlbGVhc2UgdGhlIG91dGVybW9zdCBwaG9zcGhhdGUgZ3JvdXA/[Qq]
[f]IE5vLiBUbyBjb252ZXJ0IEFUUCB0byBBRFAgYW5kIHBob3NwaGF0ZSwgdGhlIG91dGVybW9zdCBwaG9zcGhhdGUgZ3JvdXAgaXMgYnJva2VuIG9mZi4gV2hpY2ggYm9uZCB3b3VsZCBoYXZlIHRvIGJyZWFrIHRvIHJlbGVhc2UgdGhlIG91dGVybW9zdCBwaG9zcGhhdGUgZ3JvdXA/
[q topic= “ATP”]Which molecule shown below is ADP?
[c]IDE=[Qq]
[c]ID I=[Qq]
[f]IE5vLiBBRFAgaXMgc2hvcnQgZm9yIOKAmGFkZW5vc2luZSBkaS1waG9zcGhhdGUu4oCZIFRoZSDigJhkaeKAmSBzdGFuZHMgZm9yIHR3byBhbmQgcmVmZXJzIHRvIHRoZSB0d28gcGhvc3BoYXRlIGdyb3Vwcy4gV2hpY2ggb2YgdGhlc2UgdHdvIG1vbGVjdWxlcyBoYXMgVFdPIHBob3NwaGF0ZSBncm91cHM/[Qq]
[f]IFllcy4gTW9sZWN1bGUgMiBpcyBBRFAsIHdoaWNoIHlvdSBjYW4gaWRlbnRpZnkgYnkgaXRzIHR3byBwaG9zcGhhdGUgZ3JvdXBzLg==
[q topic= “ATP”]Which molecule below has more stored chemical energy?
[c]ID E=[Qq]
[c]IDI=[Qq]
[f]IFllcy4gQVRQLCB3aXRoIGl0cyB0aHJlZSBwaG9zcGhhdGUgZ3JvdXBzLCBoYXMgbW9yZSBzdG9yZWQgY2hlbWljYWwgZW5lcmd5IHRoYW4gQURQLg==[Qq]
[f]IE5vLiBJbiB0aGUgQVRQLUFEUCBzeXN0ZW0sIGhhdmluZyB0aHJlZSBwaG9zcGhhdGUgZ3JvdXBzIG1lYW5zIGhhdmluZyBtb3JlIGVuZXJneSB0aGFuIGhhdmluZyB0d28uIE5leHQgdGltZSwgY2hvb3NlIEFUUCwgd2hpY2ggaGFzIHRocmVlIHBob3NwaGF0ZSBncm91cHMu
[q topic= “ATP”]When a cell needs to release a small amount of energy, it converts
[c]IEFUUCB0byBBRFAgYW 5kIHBob3NwaGF0ZQ==[Qq]
[c]IEFEUCBhbmQgcGhvc3BoYXRlIHRvIEFUUA==[Qq]
[f]IFllcy4gQ29udmVyc2lvbiBvZiBBVFAgdG8gQURQIGFuZCBwaG9zcGhhdGUgcmVsZWFzZXMgZW5lcmd5IGZvciBjZWxsdWxhciB3b3JrLg==[Qq]
[f]IE5vLiBDb252ZXJ0aW5nIEFEUCBhbmQgcGhvc3BoYXRlIHRvIEFUUCBpcyBob3cgY2VsbHMgU1RPUkUgZW5lcmd5LiBIb3cgZG8gY2VsbHMgcmVsZWFzZSBlbmVyZ3k/
[q topic= “ATP”]When a cell needs to store a small amount of energy, it converts
[c]IEFUUCB0byBBRFAgYW5kIHBob3NwaGF0ZQ==[Qq]
[c]IEFEUCBhbmQgcGhvc3 BoYXRlIHRvIEFUUA==[Qq]
[f]IE5vLiBDb252ZXJzaW9uIG9mIEFUUCB0byBBRFAgYW5kIHBob3NwaGF0ZSByZWxlYXNlcyBlbmVyZ3ku[Qq]
[f]IFllcy4gQ29udmVydGluZyBBRFAgYW5kIFBob3NwaGF0ZSB0byBBVFAgaXMgaG93IGNlbGxzIHN0b3JlIGVuZXJneS4=
[q topic= “ATP”]In this diagram of the ATP-ADP cycle, which letter represents energy release?
[c]IEE=[Qq]
[c]IE I=
[f]IE5vLiBMZXR0ZXIgQSBzaG93cyBBRFAgYW5kIFBob3NwaGF0ZSBiZWluZyBjb252ZXJ0ZWQgaW50byBBVFAsIHdoaWNoIGlzIGhvdyBjZWxscyBzdG9yZSBlbmVyZ3kgZm9yIGNlbGx1bGFyIHdvcmsu[Qq]
[f]IFllcy4gTGV0dGVyIEIgc2hvd3MgdGhlIGNvbnZlcnNpb24gb2YgQVRQIHRvIEFEUCBhbmQgcGhvc3BoYXRlLCB3aGljaCBpcyBob3cgY2VsbHMgcmVsZWFzZSBlbmVyZ3kgZm9yIGNlbGx1bGFyIHdvcmsu
[q topic= “ATP”]Which of the following best describes the flow of energy in cells performing cellular respiration?
[c]IEZvb2QgdG8gQURQIHRvIGNlbGwgd29yaw==[Qq]
[c]IGZvb2QgZGlyZWN0bHkgdG8gY2VsbCB3b3Jr[Qq]
[c]IEFUUCB0byBmb29k[Qq]
[c]IGZvb2QgdG8gQVRQIH RvIGNlbGwgd29yaw==[Qq]
[f]IE5vLiBBRFAgaXNu4oCZdCB0aGUgbW9sZWN1bGUgdGhhdOKAmXMgYnJva2VuIGRvd24gZm9yIGNlbGwgd29yay4gRmluZCBhIHBhdGggd2hlcmUgQVRQIHByZWNlZGVzIGNlbGwgd29yay4=[Qq]
[f]IE5vLiBDZWxscyBoYXZlIHRvIGNvbnZlcnQgZm9vZCBlbmVyZ3kgdG8gQVRQIGJlZm9yZSB0aGV5IGNhbiBwZXJmb3JtIHdvcmsuIEZpbmQgYSBwYXRoIHdoZXJlIEFUUCBwcmVjZWRlcyBjZWxsIHdvcmsu[Qq]
[f]IE5vLiBJbiBjZWxsdWxhciByZXNwaXJhdGlvbiwgZm9vZCBpcyB1c2VkIHRvIG1ha2UgQVRQLiBGaW5kIGEgcGF0aCB3aGVyZSBBVFAgcHJlY2VkZXMgY2VsbCB3b3JrLg==[Qq]
[f]IFllcy4gRm9yIGNlbGxzIHRvIGhhdmUgdGhlIHR5cGUgb2YgZW5lcmd5IHRoZXkgbmVlZCB0byBwZXJmb3JtIHdvcmssIHRoZXkgbXVzdCBmaXJzdCBjb252ZXJ0IHRoZSBjaGVtaWNhbCBlbmVyZ3kgaW4gZm9vZCBpbnRvIHRoZSBjaGVtaWNhbCBlbmVyZ3kgaW4gQVRQLCB3aGljaCB0aGV5IGNhbiB0aGVuIHVzZSB0byBwZXJmb3JtIGNlbGx1bGFyIHdvcmsu
[x]
[restart]
[/qwiz]
Next Moves
-
- Cellular Respiration Overview (the next tutorial in this series)
- Cellular Respiration Main Menu
