Click the following link for a Biochemistry Student Learning Guide
Page Outline
- The Four Types of Lipids
- Lipids are Hydrophobic
- Fats and Oils
- Fats and Oils Quiz
- Phospholipids
- Steroids
- Waxes
- Lipids Quiz
1. The four types of lipids
Let’s start by organizing the four types of lipids into the concept map below. Just use prior knowledge and trial and error, and you’ll get it. Think, for example, about why some people like to wax their cars. Or remember back to the steroid scandals in Major League Baseball…
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Lipids Concept Map (HS,v2)”]
[h]Interactive Concept Map: The Four Types of Lipids
[q labels = “top”]
[l]waterproofing
[fx] No. Please try again.
[f*] Great!
[l]membranes
[fx] No. Please try again.
[f*] Excellent!
[l]fats and oils
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]insulation
[fx] No. Please try again.
[f*] Good!
[l]steroids
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[/qwiz]
2. Lipids are (mostly) hydrophobic
Lipids are up a diverse group of biomolecules. What puts fats and oils; phospholipids; steroids; and waxes into this one biomolecule family? It has to do with how they interact with water.

If you’ve spent any time in the kitchen, you know that oil and water don’t mix. That’s one of the key properties of lipids. The word we use in biology (or chemistry) to describe this is hydrophobic, which means “water-fearing.” Substances that are hydrophobic don’t dissolve in water. The molecules that are classified as lipids are either completely hydrophobic, or they have large hydrophobic regions.
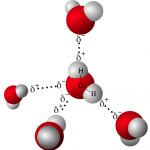
To understand why lipids are hydrophobic, we need to briefly review water’s chemistry. Water (as we discussed in a previous tutorial) is a polar molecule. Polar molecules have unequal electron sharing, which results in the molecule having partially positively charged (designated as δ+ in the diagram to your right) and partially negatively charged (δ–) regions. These charged regions interact with one another in one of two ways: similarly charged regions repel one another, and oppositely charged regions attract one another. The attraction between positively and negatively charged regions of water molecules is called a hydrogen bond (indicated by the dotted lines in the diagram to the right).
Hydrophobic molecules don’t dissolve in water. That’s because they’re non-polar. To understand this, let’s compare a molecule that does dissolve (glucose) with one that doesn’t (fat).
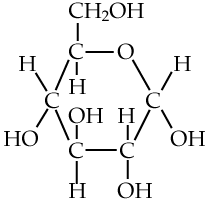
Here’s glucose. Notice all of the —OH subunits? Each one of those is polar, and will form hydrogen bonds with water molecules, enabling glucose to dissolve.
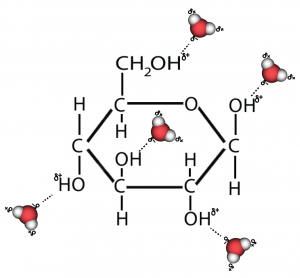
In your mind’s eye, imagine something like what I’ve drawn to the right. That’s what happens when glucose (or any other sugar) dissolves in water.
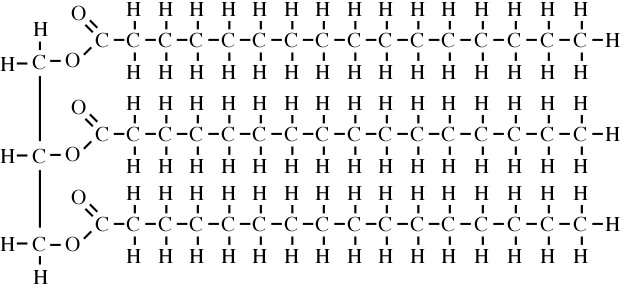 Now here’s a structural formula of a molecule of fat. Notice that it doesn’t have any of those —OH groups. As a result, there’s nothing for water to grab onto.
Now here’s a structural formula of a molecule of fat. Notice that it doesn’t have any of those —OH groups. As a result, there’s nothing for water to grab onto.
So, when you see oil droplets in water like this:
You should imagine this:
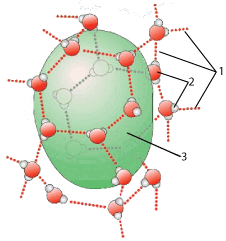
The green blob (at “3”) is a droplet of hydrophobic fat molecules clustering together because of their own very weak attraction to one another. At the same time these hydrophobic molecules are prevented from dissolving in the surrounding solution of water molecules (two of which are indicated by “2”) because they don’t form hydrogen bonds (at “1”) with the water molecules in the solution.
Got it? The quiz below will check your understanding of the four types of lipids (introduced in the concept map above), and the hydrophobic nature of lipids.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Introducing Lipids (HS,v2)”]
[h] Introducing Lipids: Checking understanding
[q] The image below shows a layer of wax (indicated by number 1) on the upper and lower surface of a leaf. As we learned above, the function of the wax is to help make the leaf [hangman]proof.
[c]IHdhdGVy[Qq]
[f]IEdyZWF0IQ==[Qq]
[q] The image below shows one of the key roles that another type of lipid — a phospholipid — plays in cells. That role is to make up the cell’s [hangman].
[c]IG1lbWJyYW5l[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[q] In warm-blooded animal like a whale that lives in cold water, one of the key functions of fat is [hangman].
[c]IGluc3VsYXRpb24=[Qq]
[f]IENvcnJlY3Qh[Qq]
[q] Steroids are lipids that act as [hangman]. Hint: testosterone and estrogen are well-known examples.
[c]IGhvcm1vbmVz[Qq]
[f]IEdvb2Qh[Qq]
[q] Let’s see if you can remember these key facts about lipids.
- The lipids that make up membranes are [hangman].
- Oils and [hangman] are used for insulation and energy storage.
- [hangman] are lipids that act as hormones.
- [hangman] is a lipid that’s used for waterproofing.
[c]IHBob3NwaG9saXBpZHM=[Qq]
[f]IEdyZWF0IQ==[Qq]
[c]IGZhdHM=[Qq]
[f]IEdyZWF0IQ==[Qq]
[c]IHN0ZXJvaWRz[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[c]IHdheA==[Qq]
[f]IEdyZWF0IQ==[Qq]
[q] One of the key features that define lipids is that they don’t dissolve in [hangman]. Another way to say that is that they’re [hangman].
[c]IHdhdGVy[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IGh5ZHJvcGhvYmlj[Qq]
[f]IEdvb2Qh[Qq]
[q] lThe diagram below is showing how glucose, which is a [hangman] molecule, can [hangman] in water. That’s because all of those “—OH” subunits can form [hangman] bonds with water molecules.
[c]IHBvbGFy[Qq]
[f]IEdvb2Qh[Qq]
[c]IGRpc3NvbHZl[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[c]IGh5ZHJvZ2Vu[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[q] In the diagram below, a hydrogen bond is shown at
[textentry single_char=”true”]
[c]ID E=
[f]IEV4Y2VsbGVudCEgTnVtYmVyICYjODIyMDsxJiM4MjIxOyBzaG93cyBhIGh5ZHJvZ2VuIGJvbmQu[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBMb29rIGZvciB0aGUgYm9uZHMgYmV0d2VlbiB0aGUgd2F0ZXIgbW9sZWN1bGVzICh0d28gb2Ygd2hpY2ggYXJlIHNob3duIGF0ICYjODIyMDsyLiYjODIyMTs=[Qq]
[q] In the diagram below, a hydrophobic substance is shown at which number?
[textentry single_char=”true”]
[c]ID M=
[f]IE5pY2Ugam9iLiBOdW1iZXIgJiM4MjIwOzMmIzgyMjE7IHNob3dzIGEgaHlkcm9waG9iaWMgc3Vic3RhbmNlIHN1cnJvdW5kZWQgYnkgd2F0ZXIgbW9sZWN1bGVzIChhdCAmIzgyMjA7MiYjODIyMTspLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBMb29rIGZvciBzb21ldGhpbmcgdGhhdCYjODIxNztzIG5vdCBhYmxlIHRvIGZvcm0gaHlkcm9nZW4gYm9uZHMgd2l0aCB0aGUgc3Vycm91bmRpbmcgd2F0ZXIgbW9sZWN1bGVzLg==[Qq]
[q] The key idea of the diagram below is that the substance making up the green droplet must be [hangman]. You can tell because it’s unable to form [hangman] bonds with the surrounding [hangman] molecules.
[c]IGh5ZHJvcGhvYmlj[Qq]
[c]aHlkcm9nZW4=[Qq]
[c]d2F0ZXI=[Qq]
[/qwiz]
3. Fats and Oils
3a. The functions of fats and oils
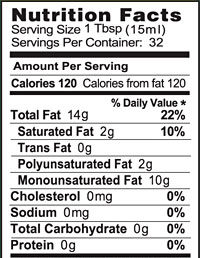
Fats and oils are the lipids that we eat. They’re the most energy-rich biomolecules. Take a moment and examine these photos and food labels for olive oil and butter.
 |
 |
|
|
|
Fats and oils both have about 9 food calories/gram. To put that in more understandable terms, a tablespoon of oil has about 120 calories, which is as many calories as are in a slice of bread.

The calorie rich nature of oil and fat explains one of their primary functions in living things: energy storage. In general, oils are synthesized by plants for energy storage, while fats are synthesized by animals (but there are plenty of exceptions). The oils in seeds (like peanut oil) store energy for the plant embryo. Animals (like bears) that hibernate store the energy from the food they’ve eaten as fat. That fat is typically found underneath the skin, but it can also be found in bone marrow or around internal organs.
This use of fat for energy storage is true of humans, too. If we take in more food energy than we need, we’ll first store it as the polysaccharide glycogen, which gets loaded into muscle and liver tissue (you can read more about this in the previous tutorial). However, our capacity to store glycogen is limited to only a small percentage of our body weight. Any additional food energy gets converted to fat (which can be stored in unlimited quantities).
Another function of fat is insulation. Think of marine mammals like whales, dolphins, seals, and sea lions; or birds such as penguins. All of these animals have thick layers of blubber, a special form of insulating fat. Blubber also serves as a source of stored energy, and makes it easier for marine animals to float. If you’re interested, you can read more about blubber here.
A special tissue called brown fat is used for controlling body temperature. The brown color comes from the fact that this tissue is loaded with mitochondria. It’s found in particularly high abundance in babies and in hibernating mammals. You can read more about it here.
3b. Fats and Oils are both Triglycerides, made from glycerol and fatty acids
Chemically, oils (like olive oil) and fats (like butter or lard) have very similar structures.
Here’s a side-by-side comparison of an oil and a fat
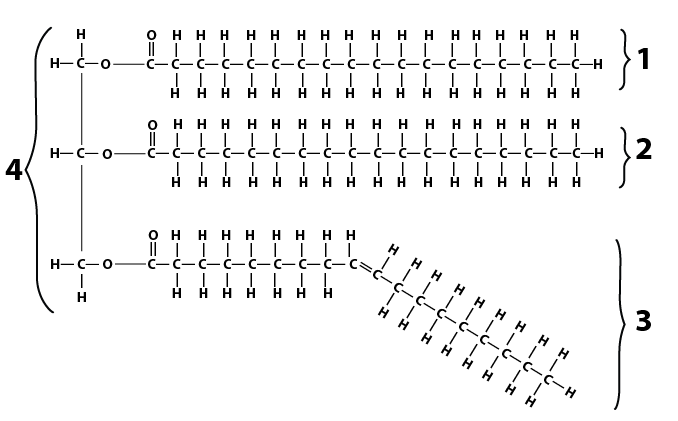 |
 |
| Oil | Fat |
Both consist of
- A 3 carbon unit called glycerol, shown at number “4” in the oil molecule above.
- Three fatty acids, shown at numbers “2,” “3” and “4” in the oil molecule.
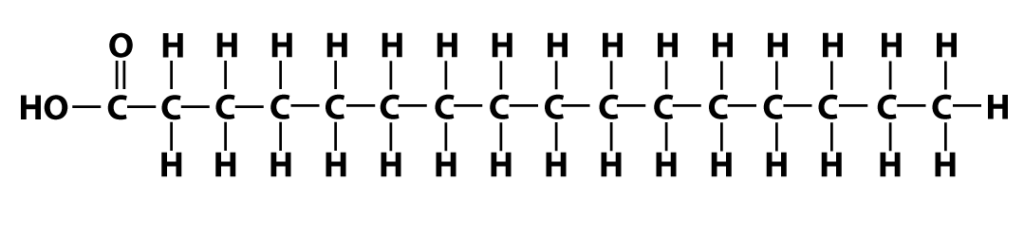 Here’s one fatty acid. Note that it has a long chain of carbons and hydrogens. The name of this kind of structure is very easy to remember: it’s a hydrocarbon chain.
Here’s one fatty acid. Note that it has a long chain of carbons and hydrogens. The name of this kind of structure is very easy to remember: it’s a hydrocarbon chain.
Hydrocarbons have lots of chemical energy stored in the chemical bonds that make them up. That’s why oils and fats are such calorie-rich foods.
Why are oils and fats different? Notably, oils are liquid at room temperature. Fats, which, you’ll find in foods such as lard (animal fat), shortening, margarine, butter and cheese are solid at room temperature. The “room temperature” part is important. It’s easy enough to get oils to solidify when you put them in a refrigerator, and all you have to do is to put butter on a frying pan to get it to liquify.
The difference is all about the fatty acids. In fats, the three fatty acids are saturated. When a paper towel, or a baby’s diaper, or kitchen sponge is saturated, it can’t hold any more water. When a fatty acid is saturated, the carbons in the hydrocarbon chain can’t bond with any more hydrogens. As a result, all of the carbons are connected to one another by single bonds (represented by a single dash between each carbon).

 Notice that in fat molecule above, the hydrocarbon chains are straight. This allows the fatty acids to form very weak bonds with one another, as well as with adjacent fat molecules. These bonds keep the resulting substances (like shortening, butter, margarine, or fat) solid at room temperature. The bonds enable them to hold their shape.
Notice that in fat molecule above, the hydrocarbon chains are straight. This allows the fatty acids to form very weak bonds with one another, as well as with adjacent fat molecules. These bonds keep the resulting substances (like shortening, butter, margarine, or fat) solid at room temperature. The bonds enable them to hold their shape.
In oils, at least one of the fatty acids within the triglyceride molecule is unsaturated, as shown in number “3” below. Note that in about the middle of this fatty acid there’s a double bond between two adjacent carbon atoms.

 The double bond in the third fatty acid causes the hydrocarbon chain to bend. Because of this bend, the fatty acids can’t bond with one another, or with other oil molecules. Because there’s no bonding, the molecules can’t hold their shape, making them liquid.
The double bond in the third fatty acid causes the hydrocarbon chain to bend. Because of this bend, the fatty acids can’t bond with one another, or with other oil molecules. Because there’s no bonding, the molecules can’t hold their shape, making them liquid.
3c. Saturated Fats, Trans fats and Cardiovascular Health
According to the American Heart Association, it’s a good idea to limit saturated fats to only a small proportion of the calories you take in. That’s because saturated fat intake seems to be connected with heart disease. The Heart Association’s recommendation is to limit saturated fats to about 13 grams/day.(source: American Heart Association) Here’s the saturated fat content of a few fatty foods.
| Food (and quantity) | Total Fat | Saturated Fat | Saturated fat density (saturated fat grams/gram of food) |
| Dry Salami (1 oz/28 grams) | 11 grams | 4 grams | 0.25 |
| Ground Beef (4 oz/113 grams) | 17 grams | 7 grams | 0.06 |
| Cheddar Cheese Stick (3/4 oz/21 grams) | 7 grams | 4.5 grams | 0.21 |
| Breakfast sausage (4 oz/113 grams) | 20 grams | 8 grams | 0.07 |
In a typical North American diet, the amount of saturated fat that a person consumes can build up pretty fast. If you had sausage for breakfast and a cheeseburger for lunch, you’d be over the American Heart Association recommendations. Note also that the type of meat you choose is important. The ground beef that I listed above is 85% lean, 15% fat. There are both fatter and leaner types of meat.
There’s also one kind of unsaturated fat to watch out for. These are called trans fats. The biochemistry of trans-fats goes beyond what’s taught in a typical high school biology course. If you’re interested, you can jump over to this tutorial in my AP Biology curriculum. You can also click here to read what the American Heart association has to say about trans fats.
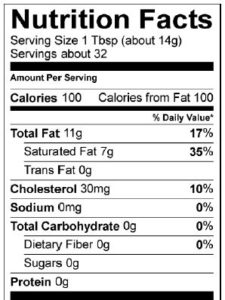
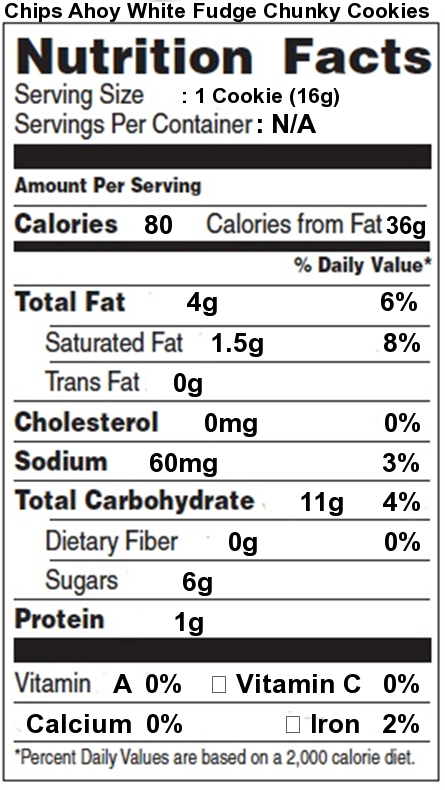 Food companies have become sensitive to consumer concerns over the connection between fat and health. Take a minute to look at the nutrition facts label for Chips Ahoy White Fudge Chunky cookies. As with almost any food with a nutrition facts label, you can see total fat, saturated fat, and trans fat.
Food companies have become sensitive to consumer concerns over the connection between fat and health. Take a minute to look at the nutrition facts label for Chips Ahoy White Fudge Chunky cookies. As with almost any food with a nutrition facts label, you can see total fat, saturated fat, and trans fat.
4. Fats and Oils: Checking Understanding
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Fats and Oils (HSv2)”]
[h]Fats and Oils: Checking Understanding
[i]
[q] Lipids are, for the most part, [hangman], which means that they won’t dissolve in water.
[c]IGh5ZHJvcGhvYmlj[Qq]
[f]IENvcnJlY3Qh
Cg==[Qq]
[q] The kind of bond that lipids won’t form with water is a [hangman] bond.
[c]IGh5ZHJvZ2Vu[Qq]
[f]IEV4Y2VsbGVudCE=
Cg==[Qq]
[q] In the diagram below, the lipid is at
[textentry single_char=”true”]
[c]ID M=[Qq]
[f]IEV4Y2VsbGVudC4gVGhlIGxpcGlkIG1vbGVjdWxlcyBhcmUgZm9ybWluZyBhIGh5ZHJvcGhvYmljIGRyb3BsZXQsIGFzIGlzIHNob3duIGF0ICYjODIyMDszLiYjODIyMTs=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50OiB0aGUgbGlwaWQgaXMgc3Vycm91bmRlZCBieSB3YXRlciBtb2xlY3VsZXMsIGFuZCBpdCB3b24mIzgyMTc7dCBmb3JtIGh5ZHJvZ2VuIGJvbmRzIHdpdGggd2F0ZXIgbW9sZWN1bGVzLg==
Cg==[Qq]
[q] In the diagram below, hydrogen bonds are indicated by number
[textentry single_char=”true”]
[c]ID E=[Qq]
[f]IEV4Y2VsbGVudC4gVGhlIGh5ZHJvZ2VuIGJvbmRzIGFyZSBpbmRpY2F0ZWQgYnkgbnVtYmVyICYjODIyMDsxLiYjODIyMTs=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBIeWRyb2dlbiBib25kcyBhcmUgYm9uZHMgYmV0d2VlbiB3YXRlciBtb2xlY3VsZXMu
Cg==[Qq]
[q] One of the key functions of fat and oil is their ability to store lots of[hangman]
[c]IGVuZXJneQ==[Qq]
[f]IEV4Y2VsbGVudCE=
Cg==[Qq]
[q] Fats and oils both can store about ____ food calories / gram
[textentry single_char=”true”]
[c]ID k=[Qq]
[f]IEV4Y2VsbGVudC4gRmF0cyBhbmQgb2lscyBjYW4gYm90aCBzdG9yZSBhYm91dCA5IGNhbG9yaWVzL2dyYW0u[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBJdCYjODIxNztzIHNpZ25pZmljYW50bHkgbW9yZSBlbmVyZ3kgdGhhbiBjYW4gYmUgc3RvcmVkIGluIGNhcmJvaHlkcmF0ZXMsIHdoaWNoIGNhbiBzdG9yZSBhYm91dCA0IGZvb2QgY2Fsb3JpZXMvZ3JhbS4=
Cg==[Qq]
[q] In addition to energy storage, the fatty blubber found in marine mammals provides them with a layer of [hangman] to protect them from heat loss.
[c]IGluc3VsYXRpb24=[Qq]
[f]IEdyZWF0IQ==
Cg==[Qq]
[q] A type of mitochondria-rich fat tissue called [hangman]fat is used for regulating body temperature in babies and in hibernating mammals.
[c]IGJyb3du[Qq]
[f]IENvcnJlY3Qh
Cg==[Qq]
[q] A chemical name for both fats and oils is [hangman].
[c]IHRyaWdseWNlcmlkZQ==[Qq]
[f]IEdyZWF0IQ==
Cg==[Qq]
[q] Triglycerides have ___ fatty acids bonded to one molecule of glycerol. (Note: type in a digit, 0 through 9)
[textentry single_char=”true”]
[c]ID M=[Qq]
[f]IEV4Y2VsbGVudC4gVHJpZ2x5Y2VyaWRlcyBoYXZlIHRocmVlIGZhdHR5IGFjaWRzIGJvbmRlZCB0byBvbmUgbW9sZWN1bGUgb2YgZ2x5Y2Vyb2wu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBodWdlIGhpbnQuIFRoZSBwcmVmaXggJiM4MjIwO3RyaSYjODIyMTsgc3RhbmRzIGZvciAmIzgyMzA7Pw==
Cg==[Qq]
[q] Of the images shown below, which one shows a saturated fatty acid?
[textentry single_char=”true”]
[c]ID E=[Qq]
[f]IEV4Y2VsbGVudC4gJiM4MjIwOzEmIzgyMjE7IHNob3dzIGEgc2F0dXJhdGVkIGZhdHR5IGFjaWQsIHdpdGggaXRzIGh5ZHJvY2FyYm9uIGNoYWluIGZpbGxlZCB0byBjYXBhY2l0eS4=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBSZW1lbWJlciB0aGF0IHNhdHVyYXRlZCBtZWFucyAmIzgyMjA7ZmlsbGVkIHRvIGNhcGFjaXR5LiYjODIyMTsgV2hpY2ggb2YgdGhlc2UgZmF0dHkgYWNpZHMgaGFzIGEgaHlkcm9jYXJib24gY2hhaW4gdGhhdCBpcyBmaWxsZWQgdG8gY2FwYWNpdHkgd2l0aCBoeWRyb2dlbi4=[Qq]
[q] Saturated fats are usually [hangman] at room temperature.
[c]IHNvbGlk
Cg==[Qq]
[q] Unsaturated triglycerides (also known as “oils”) are usually [hangman] at room temperature
[c]IGxpcXVpZA==
Cg==[Qq]
[x][restart][/qwiz]
5. Phospholipids
Phospholipids are the key components of cell membranes.
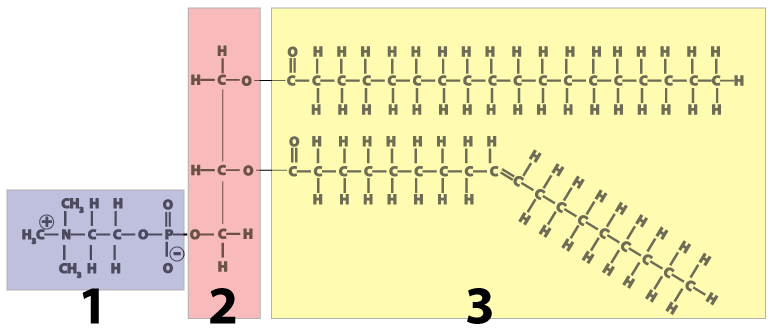
- Like triglycerides (fats and oils), phospholipids are built around a molecule of glycerol, a 3-carbon alcohol, (shown in pink at number 2 in the diagram).
- Attached to the glycerol on one side are two fatty acids (shown in yellow at number 3). The two fatty acids make up the molecule’s “tail.”
- On the other side of the molecule (number 1 in the diagram) is a phosphate-bearing “head.”
Typically, phospholipids are represented by diagrams like the one shown on the right. Whatever orientation you see this in, you should immediately be able to recognize it as a phospholipid.
In terms of how phospholipids interact with water, they have have a split personality.
- The fatty acid tails are non-polar, making them hydrophobic (water fearing). In other words, they act like a fat or an oil, avoiding water.
- The head is polar. This makes the head hydrophilic (water-loving). In other words, the head interacts with water through forming hydrogen bonds with whatever water molecules it bumps into.
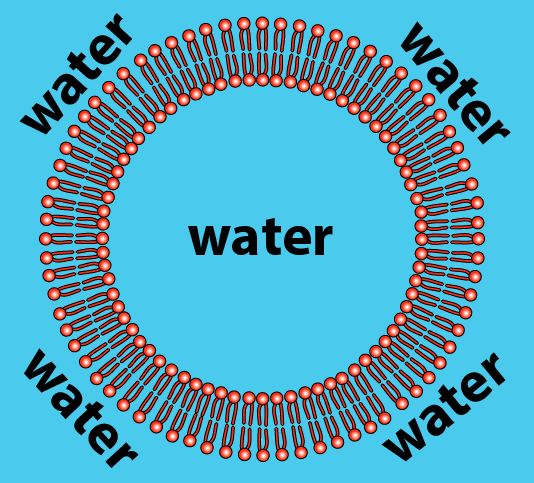
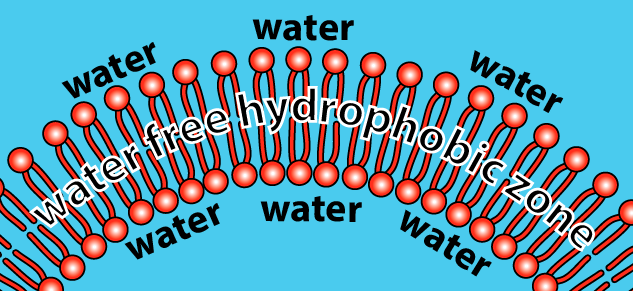
As a result, phospholipids, when mixed with water, spontaneously organize themselves into a few orientations. The one you need to understand is called a bilayer. The bilayer is a membrane that defines a sphere (which you can think of as an empty cell). If we sliced open the sphere, here’s what we’d see:
How does this work? Let’s just look at a tiny slice of the sphere.
- The hydrophilic heads of the phospholipids orient themselves so that the heads are in contact with water.
- The hydrophobic tails cluster together. This is the same clustering that happens in a droplet of oil mixed in water. All of the tails create a hydrophobic, water-free zone.
You’ll learn more about phospholipids in the context of membranes. For now, let’s consolidate our understanding with this quiz.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Introducing Phospholipids (HS,v2)”]
[h]Phospholipids
[i]
[q]The molecules that make up that basic structure of the membrane are called [hangman].
[c]cGhvc3Bob2xpcGlkcw==[Qq]
[q]The two fatty acid chains make up the phospholipid’s [hangman].
[c]dGFpbA==
Cg==[Qq]
[q]The phosphate containing part makes up the phospholipid’s [hangman].
[c]aGVhZA==
Cg==[Qq]
[q]The “tail” of a phospholipid is “water-fearing,” or [hangman].
[c]aHlkcm9waG9iaWM=[Qq]
[q]The “head” of a phospholipid is “water-loving” or [hangman]
[c]aHlkcm9waGlsaWM=
Cg==W2FdQSBtb3JlIHNjaWVudGlmaWMgd2F5IHRvIHNheSAmIzgyMjA7d2F0ZXItbG92aW5nJiM4MjIxOyBpcyA=aHlkcm9waGlsaWM=Lg==[Qq]
[q]The tail of a phospholipid is
[c]bm9ucG 9sYXI=[Qq]
[f]Q29ycmVjdC4gQSBwaG9zcGhvbGlwaWQmIzgyMTc7cyB0YWlsIGlzIG5vbnBvbGFyIGFuZCBoeWRyb3Bob2JpYw==[Qq]
[c]cG9sYXI=[Qq]
[f]Tm8uIEJlY2F1c2Ugb2YgdGhlIGxvbmcgaHlkcm9jYXJib24gY2hhaW5zLCB0aGUgdGFpbCBvZiBhIHBob3NwaG9saXBpZCBpcyBoeWRyb3Bob2JpYy4=[Qq]
[q]The arrangement of phospholipids shown below is called a phospholipid [hangman]
[c]YmlsYXllcg==[Qq]
[x]
If you want more practice, please press the restart button below. Otherwise, follow the links below.
[restart]
[/qwiz]
6. Steroids
The molecule below is testosterone, an example of a type of lipid called a steroid.
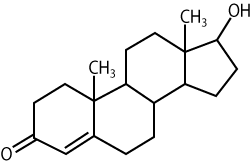
Steroids are all built around four fused carbon rings. What makes one steroid different from another are variations in this carbon skeleton (for example, the position of double bonds) along with attachment of various chemical groups. See, for example, if you can find the difference between testosterone and the steroid molecule below.
[qwiz style=”width: 600px !important;”]
[h]Analyzing a steroid structural formula
[q]
[c]IENsaWNrIHdoZW4geW91JiM4MjE3O3JlIH JlYWR5IHRvIHNlZSB0aGUgYW5zd2VyLg==[Qq]
[f]
Cg==| [Qq]
|
|
The molecule on the left is estrogen. Compared to testosterone, estrogen has 1) two more double bonds in the carbon ring on the bottom left, 2) no methyl group(–CH3) sticking up between the two bottom carbon rings, and 3) a hydroxyl functional group (–OH) instead of a carbonyl group (=O) sticking out from the lower left. Those three differences, though, are enough to account for the difference between females and males!
[/qwiz]
As you can see from the example of testosterone and estrogen, one function of steroids is to act as hormones. Hormones are molecules that are produced in a gland in one part of the body, get released into the bloodstream, and, in the case of steroid hormones, enter into cells and cause changes in cellular activity (there are other types of hormones that don’t enter into cells, but which bind at the membrane).
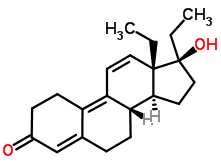
If you’re a sports fan, you’re probably aware of athletes who have been accused of and/or found guilty of using steroids to increase their athletic performance. These drugs are called anabolic steroids. You can think of them as artificial forms of testosterone, and they have the same effect on the body as testosterone has: increasing muscle mass, which increases strength and performance. On such performance-enhancing anabolic steroid is tetrahydrogestrinone, which was used by baseball player Barry Bonds (knowingly or not: you can read about the controversy here).
A steroid with a different role is cholesterol.
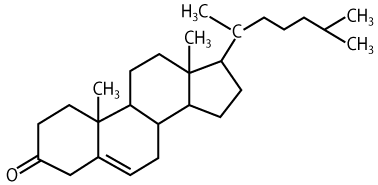
Cholesterol is found in cell membranes, where it keeps membrane molecules like phospholipids and proteins fluid (which is important for allowing diffusion of substances across membranes), Cholesterol also maintains membrane strength, which is important in animal cells, which lack cell walls. Cholesterol, which is synthesized primarily by the liver, is also a starting point for biosynthesis of other steroid molecules, including the steroid hormones discussed above.
Cholesterol, along with the saturated fats and trans-fats discussed above, can negatively impact cardiovascular health. Click the link that follows to read what the American Heart Association has to say about cholesterol and heart disease.
7. Waxes
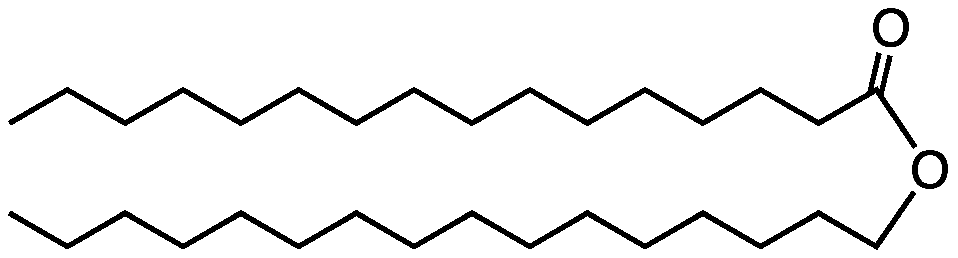
Waxes are another type of lipid. Looking at the structural formula below (and remembering that you have to imagine a carbon and hydrogen atoms at every angle vertex), you can see that waxes have long hydrocarbon chains. That’s why candle wax has a lot of energy.
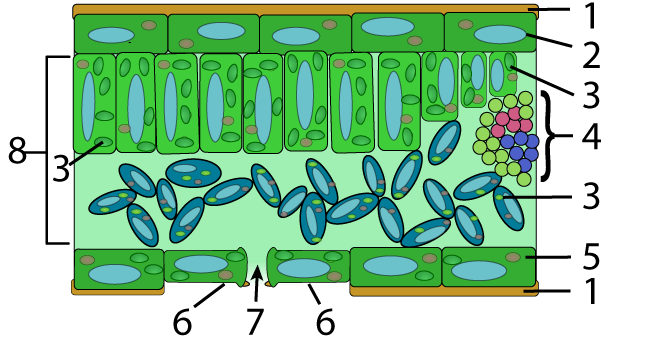
In plants, waxes are found on the upper surfaces of leaves, where they prevent water loss. The diagram of a leaf cross section below shows this waxy layer (also called a “cuticle”) at number “1,” on both the upper and lower surface of the leaf.
In the animal world, bees use wax to construct their honeycombs. Worker bees synthesize this wax in wax-producing glands in their abdomens that convert the sugar in honey into wax.

As with fats, bonds between the hydrocarbon chains within wax molecules and between wax molecules make waxes solid. And while the melting point of waxes is higher than that of fats like lard or margarine, it’s not that high (as anyone who has ever lit a wax candle knows).
8. Lipids: Checking Understanding
Here’s just a few questions about lipids for review.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Lipids, cumulative quiz (HS,v2)”]
[h]
Lipids
[i]
[q] The molecule shown below is a
[c]IGZhdHR5IGFjaWQ=[Qq]
[f]IE5vLiBGYXR0eSBhY2lkcyBoYXZlIGEgbG9uZyBoeWRyb2NhcmJvbiB0YWlsLCBhdHRhY2hlZCB0byBhIGNhcmJveHlsIGdyb3VwLiBOb3RlIHRoZSBmb3VyIGZ1c2VkIGNhcmJvbiByaW5ncy4=
Cg==[Qq]
[c]IHRyaWdseWNlcmlkZQ==[Qq]
[f]IE5vLiBUcmlnbHljZXJpZGVzIGhhdmUgdGhyZWUgZmF0dHkgYWNpZHMgYXR0YWNoZWQgdG8gb25lIGdseWNlcm9sLg==[Qq]
[c]IHdheA==[Qq]
[f]IE5vLiBXYXhlcyB1c3VhbGx5IGNvbnNpc3Qgb2YgdHdvIGxvbmcgaHlkcm9jYXJib24gY2hhaW5z
Cg==[Qq]
[c]IHN0ZX JvaWQ=[Qq]
[f]IEV4Y2VsbGVudC4gVGhpcyBtb2xlY3VsZSBpcyBhIHN0ZXJvaWQgKHNwZWNpZmljYWxseSwgaXQmIzgyMTc7cyB0aGUgaG9ybW9uZSBlc3Ryb2dlbik=[Qq]
[c]IHBob3NwaG9saXBpZA==[Qq]
[f]IE5vLiBQaG9zcGhvbGlwaWRzIGNvbnNpc3Qgb2YgdHdvIG5vbi1wb2xhciBmYXR0eSBhY2lkIGNoYWlucywgYXR0YWNoZWQgdG8gYSBnbHljZXJvbCwgd2hpY2ggaXMgYXR0YWNoZWQgdG8gYSBwb2xhciAmIzgyMjA7aGVhZCYjODIyMTsgY29uc2lzdGluZyBvZiBhIHBob3NwaGF0ZSBncm91cA==
Cg==[Qq]
[q] The molecule shown below is a
[c]IGZhdHR5 IGFjaWQ=[Qq]
[f]IEV4Y2VsbGVudCEgVGhpcyBtb2xlY3VsZSBpcywgaW5kZWVkLCBhIGZhdHR5IGFjaWQuIC4=[Qq]
[c]IHRyaWdseWNlcmlkZQ==[Qq]
[f]IE5vLiBUcmlnbHljZXJpZGVzIGhhdmUgdGhyZWUgZmF0dHkgYWNpZHMgYXR0YWNoZWQgdG8gb25lIGdseWNlcm9sLg==[Qq]
[c]IHdheA==[Qq]
[f]IE5vLiBXYXhlcyBjb25zaXN0IG9mIHR3byBsb25nIGh5ZHJvY2FyYm9uIGNoYWlucywgYXQgbGVhc3Qgb25lIG9mIHdoaWNoIGlzIGEgZmF0dHkgYWNpZC4=
Cg==[Qq]
[c]IHN0ZXJvaWQ=[Qq]
[f]IE5vLiBTdGVyb2lkcyBjb25zaXN0IG9mIGZvdXIgZnVzZWQgY2FyYm9uIHJpbmdzLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[c]IHBob3NwaG9saXBpZA==[Qq]
[f]IE5vLiBQaG9zcGhvbGlwaWRzIGNvbnNpc3Qgb2YgdHdvIG5vbi1wb2xhciBmYXR0eSBhY2lkcyBjaGFpbiwgYXR0YWNoZWQgdG8gYSBnbHljZXJvbCwgd2hpY2ggaXMgYXR0YWNoZWQgdG8gYSBwb2xhciAmIzgyMjA7aGVhZCYjODIyMTsgY29uc2lzdGluZyBvZiBhIHBob3NwaGF0ZSBncm91cC4=
Cg==[Qq]
[q] The molecule shown below is a
[c]IGZhdHR5IGFjaWQ=[Qq]
[f]IE5vLiBUaGlzIG1vbGVjdWxlIGNvbnRhaW5zIGZhdHR5IGFjaWRzICh0aHJlZSBvZiB0aGVtLCBpbiBmYWN0KSwgYnV0IGl0JiM4MjE3O3Mgbm90IGp1c3QgYSBmYXR0eSBhY2lkLiBBIHNpbmdsZSBmYXR0eSBhY2lkIGlzIHNob3duIGJlbG93
Cg==[Qq]
[c]IHRyaWdseW NlcmlkZQ==[Qq]
[f]IE5pY2Ugam9iLiBUcmlnbHljZXJpZGVzIGhhdmUgdGhyZWUgZmF0dHkgYWNpZHMgYXR0YWNoZWQgdG8gb25lIGdseWNlcm9sLg==
Cg==[Qq]
[c]IHdheA==[Qq]
[f]IE5vLiBXYXhlcyBjb25zaXN0IG9mIHR3byBsb25nIGh5ZHJvY2FyYm9uIGNoYWlucywgYXQgbGVhc3Qgb25lIG9mIHdoaWNoIGlzIGEgZmF0dHkgYWNpZC4=
Cg==[Qq]
[c]IHN0ZXJvaWQ=[Qq]
[f]IE5vLiBTdGVyb2lkcyBjb25zaXN0IG9mIGZvdXIgZnVzZWQgY2FyYm9uIHJpbmdzLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[c]IHBob3NwaG9saXBpZA==[Qq]
[f]IE5vLiBQaG9zcGhvbGlwaWRzIGNvbnNpc3Qgb2YgdHdvIG5vbi1wb2xhciBmYXR0eSBhY2lkcyBjaGFpbiwgYXR0YWNoZWQgdG8gYSBnbHljZXJvbCwgd2hpY2ggaXMgYXR0YWNoZWQgdG8gYSBwb2xhciAmIzgyMjA7aGVhZCYjODIyMTsgY29uc2lzdGluZyBvZiBhIHBob3NwaGF0ZSBncm91cC4=
Cg==[Qq]
[q] The molecule shown below is a
[c]IGZhdHR5IGFjaWQ=[Qq]
[f]IE5vLiBUaGlzIG1vbGVjdWxlIGNvbnRhaW5zIGZhdHR5IGFjaWRzICh0d28gb2YgdGhlbSwgaW4gZmFjdCksIGJ1dCBpdCYjODIxNztzIG5vdCBqdXN0IGEgZmF0dHkgYWNpZC4gQSBzaW5nbGUgZmF0dHkgYWNpZCBpcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[c]IHRyaWdseWNlcmlkZQ==[Qq]
[f]IE5vLiBUcmlnbHljZXJpZGVzIGhhdmUgdGhyZWUgZmF0dHkgYWNpZHMgYXR0YWNoZWQgdG8gb25lIGdseWNlcm9sLg==[Qq]
[c]IHdheA==[Qq]
[f]IE5vLiBXYXhlcyBjb25zaXN0IG9mIHR3byBsb25nIGh5ZHJvY2FyYm9uIGNoYWlucywgYXQgbGVhc3Qgb25lIG9mIHdoaWNoIGlzIGEgZmF0dHkgYWNpZC4=
Cg==[Qq]
[c]IHN0ZXJvaWQ=[Qq]
[f]IE5vLiBTdGVyb2lkcyBjb25zaXN0IG9mIGZvdXIgZnVzZWQgY2FyYm9uIHJpbmdzLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[c]IHBob3NwaG 9saXBpZA==[Qq]
[f]IEV4Y2VsbGVudC4gUGhvc3Bob2xpcGlkcyBjb25zaXN0IG9mIHR3byBub24tcG9sYXIgZmF0dHkgYWNpZHMgY2hhaW4sIGF0dGFjaGVkIHRvIGEgZ2x5Y2Vyb2wsIHdoaWNoIGlzIGF0dGFjaGVkIHRvIGEgcG9sYXIgJiM4MjIwO2hlYWQmIzgyMjE7IGNvbnNpc3Rpbmcgb2YgYSBwaG9zcGhhdGUgZ3JvdXAu[Qq]
[q] A molecule that’s the key building block of cell membranes.
[c]IHN0ZXJvaWQ=[Qq]
[f]IE5vLiBXaGlsZSBjaG9sZXN0ZXJvbCBpcyBhIHN0ZXJvaWQgdGhhdCYjODIxNztzIGEgcGFydCBvZiBjZWxsIG1lbWJyYW5lcywgaXQmIzgyMTc7cyBub3QgdGhlIG1haW4gY29tcG9uZW50LiBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3Igc29tZXRoaW5nIHRoYXQgb3JnYW5pemVzIGl0c2VsZiBpbnRvIGEgYmlsYXllci4=[Qq]
[c]IHRyaWdseWNlcmlkZQ==[Qq]
[f]IE5vLiBUcmlnbHljZXJpZGVzIGFyZSB1c2VkIGZvciBlbmVyZ3kgc3RvcmFnZSwgaW5zdWxhdGlvbiwgYW5kIGJ1b3lhbmN5LiBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3Igc29tZXRoaW5nIHRoYXQgb3JnYW5pemVzIGl0c2VsZiBpbnRvIGEgYmlsYXllci4=[Qq]
[c]IHBob3NwaG 9saXBpZA==[Qq]
[f]IEV4Y2VsbGVudC4gUGhvc3Bob2xpcGlkcyBhcmUgdGhlIGtleSBidWlsZGluZyBibG9ja3Mgb2YgY2VsbCBtZW1icmFuZXMu[Qq]
[c]IHdheA==[Qq]
[f]IE5vLiBXYXhlcyBhcmUgdXNlZCBmb3Igd2F0ZXJwcm9vZmluZy4gWW91JiM4MjE3O3JlIGxvb2tpbmcgZm9yIGEgbW9sZWN1bGUgdGhhdCBvcmdhbml6ZXMgaXRzZWxmIGludG8gYSBiaWxheWVyLg==[Qq]
[q] A molecule that’s used for energy storage, insulation, and buoyancy.
[c]IHN0ZXJvaWQ=[Qq]
[f]IE5vLiBTdGVyb2lkcyBpbmNsdWRlIGhvcm1vbmVzIGxpa2UgdGVzdG9zdGVyb25lIGFuZCBlc3Ryb2dlbiwgYW5kIGFsc28gbW9sZWN1bGVzIGxpa2UgY2hvbGVzdGVyb2wsIGEga2V5IGNvbXBvbmVudCBvZiBjZWxsIG1lbWJyYW5lcy4=[Qq]
[c]IHRyaWdseW NlcmlkZQ==[Qq]
[f]IEV4Y2VsbGVudCEgVHJpZ2x5Y2VyaWRlcyBpbmNsdWRlIGZhdHMgYW5kIG9pbHMsIGFuZCBhcmUgdXNlZCBmb3IgZW5lcmd5IHN0b3JhZ2UsIGluc3VsYXRpb24sIGFuZCBidW95YW5jeS4=[Qq]
[c]IHBob3NwaG9saXBpZA==[Qq]
[f]IE5vLiBQaG9zcGhvbGlwaWRzIGFyZSB0aGUga2V5IGJ1aWxkaW5nIGJsb2NrcyBvZiBjZWxsIG1lbWJyYW5lcy4=[Qq]
[c]IHdheA==[Qq]
[f]IE5vLiBXYXhlcyBhcmUgdXNlZCBmb3Igd2F0ZXJwcm9vZmluZy4gWW91JiM4MjE3O3JlIGxvb2tpbmcgZm9yIHRoZSB0eXBlIG9mIG1vbGVjdWxlIHRoYXQgbWFrZXMgdXAgZmF0cyBhbmQgb2lscy4=[Qq]
[q] The type of lipid that forms certain hormones, as well as the membrane component cholesterol.
[c]IHN0ZX JvaWQ=[Qq]
[f]IEF3ZXNvbWUhIFN0ZXJvaWRzIGluY2x1ZGUgaG9ybW9uZXMgbGlrZSB0ZXN0b3N0ZXJvbmUgYW5kIGVzdHJvZ2VuLCBhbmQgYWxzbyBtb2xlY3VsZXMgbGlrZSBjaG9sZXN0ZXJvbCwgYSBrZXkgY29tcG9uZW50IG9mIGNlbGwgbWVtYnJhbmVzLg==[Qq]
[c]IHRyaWdseWNlcmlkZQ==[Qq]
[f]IE5vLiBUcmlnbHljZXJpZGVzIGluY2x1ZGUgZmF0cyBhbmQgb2lscywgYW5kIGFyZSB1c2VkIGZvciBlbmVyZ3kgc3RvcmFnZSwgaW5zdWxhdGlvbiwgYW5kIGJ1b3lhbmN5Lg==[Qq]
[c]IHBob3NwaG9saXBpZA==[Qq]
[f]IE5vLiBQaG9zcGhvbGlwaWRzIGFyZSB0aGUga2V5IGJ1aWxkaW5nIGJsb2NrcyBvZiBjZWxsIG1lbWJyYW5lcy4=[Qq]
[c]IHdheA==[Qq]
[f]IE5vLiBXYXhlcyBhcmUgdXNlZCBmb3Igd2F0ZXJwcm9vZmluZy4=[Qq]
[q] A molecule used for waterproofing.
[c]IHN0ZXJvaWQ=[Qq]
[f]IE5vLlN0ZXJvaWRzIGluY2x1ZGUgaG9ybW9uZXMgbGlrZSB0ZXN0b3N0ZXJvbmUgYW5kIGVzdHJvZ2VuLCBhbmQgYWxzbyBtb2xlY3VsZXMgbGlrZSBjaG9sZXN0ZXJvbCwgYSBrZXkgY29tcG9uZW50IG9mIGNlbGwgbWVtYnJhbmVzLg==[Qq]
[c]IHRyaWdseWNlcmlkZQ==[Qq]
[f]IE5vLiBUcmlnbHljZXJpZGVzIGluY2x1ZGUgZmF0cyBhbmQgb2lscywgYW5kIGFyZSB1c2VkIGZvciBlbmVyZ3kgc3RvcmFnZSwgaW5zdWxhdGlvbiwgYW5kIGJ1b3lhbmN5Lg==[Qq]
[c]IHBob3NwaG9saXBpZA==[Qq]
[f]IE5vLiBQaG9zcGhvbGlwaWRzIGFyZSB0aGUga2V5IGJ1aWxkaW5nIGJsb2NrcyBvZiBjZWxsIG1lbWJyYW5lcy4=[Qq]
[c]IHdh eA==[Qq]
[f]IEV4Y2VsbGVudC4gV2F4ZXMgYXJlIHVzZWQgZm9yIHdhdGVycHJvb2Zpbmcu[Qq]
[x][restart]
[/qwiz]