1. Monosaccharides and Disaccharides
1a. Monosaccharides are the simplest sugars
Carbohydrates consist of sugars, starches, and plant fiber.
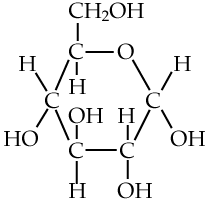
The simplest carbohydrates are simple sugars, also known as monosaccharides. “Monosaccharide” means “one sugar,” which will make more sense below when we see these simple sugars — like the molecule of glucose shown at left —combined together to form larger and more complex molecules.
Glucose is a monosaccharide that you should know (at least by name). It’s the molecule that plants make during photosynthesis. Here’s a word equation for photosynthesis
PHOTOSYNTHESIS: carbon dioxide + light energy → glucose + oxygen
Glucose is also what the cells of both plants and animals use for energy during cellular respiration:
CELLULAR RESPIRATION: glucose + oxygen → carbon dioxide + water + ATP
ATP, as we’ll learn about later, is the molecule that cells use to do the work of staying alive.
When we eat other carbohydrates (such as potatoes, bread, or rice), our digestive system breaks them down to glucose. Glucose, as a result is the sugar that circulates in our bloodstream. The disease diabetes is caused by an inability to control blood glucose levels.
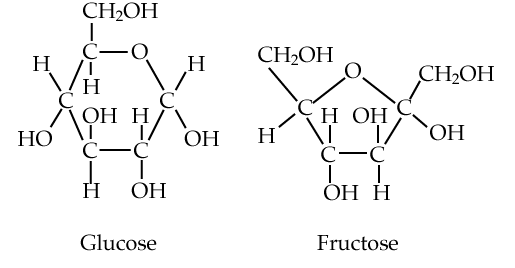 Another monosaccharide is fructose, shown right next to glucose in the diagram at left. Fructose is commonly used as a sweetener in soft drinks. Take in too much of this monosaccharide, and you could be setting your self up for heart problems and diabetes (source: Cleveland Clinic).
Another monosaccharide is fructose, shown right next to glucose in the diagram at left. Fructose is commonly used as a sweetener in soft drinks. Take in too much of this monosaccharide, and you could be setting your self up for heart problems and diabetes (source: Cleveland Clinic).
Continue by answering the questions below.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-monosaccharides (HS)”]
[h]Monosaccharides
[i]
[q]These two molecules might look pretty different, but they have one important feature in common. Take a moment to study the diagram below, then click “continue”
[q labels =”top”]Both glucose and fructose have the same number of carbons, hydrogens, and oxygens. Drag the numbers into the right place
C__H__O__
[l]6
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]12
[fx] No. Please try again.
[f*] Good!
[q]Here’s another similarity. Both glucose and fructose are composed of the same three elements. In alphabetical order, these are [hangman], [hangman] and [hangman].
[c]Y2FyYm9u[Qq]
[c]aHlkcm9nZW4=[Qq]
[c]b3h5Z2Vu[Qq]
[q]Let’s finish this quiz by thinking about the chemical formula for both glucose and fructose. As we’ve discovered, it’s C6H12O6. Let’s think about the relationship between the number of hydrogen atoms and the number of oxygen atoms. In both glucose and fructose, there are [hangman] as many hydrogens as there are oxygens.
[c]dHdpY2U=[Qq]
[x]
[/qwiz]
The two to one ratio of hydrogen to oxygen is found in pretty much every carbohydrate. In fact, carbohydrate means “hydrate” of carbon.What does that mean? When you’re hydrated, you have enough water. When carbon atoms are bonded to hydrogen and oxygen in a 2:1 ratio, they’re considered to be hydrated: that’s the origin of the term “carbohydrate.”
1b. Disaccharides consists of two, linked monosaccharides
Monosaccharides are the monomers of carbohydrates. That means that they’re the building blocks from which other carbohydrates are built. When plant cells take two monosaccharides and link them together, they form disaccharides. Disaccharide means “double sugar.”
Here’s a double sugar that you’re probably familiar with: sucrose, also known as table sugar.
 |
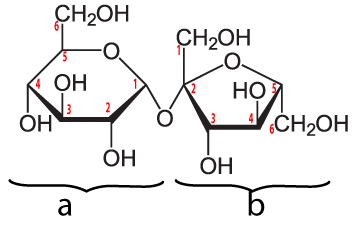 |
Sucrose is a disaccharide. Its structural formula is shown above. This formula might seem a little weird, because uses a convention that biochemists use. Every place where there’s an angle, you have to imagine that there’s a carbon atom, along with attached hydrogens. Also, you can ignore the little red numbers next to each carbon).
Sucrose is formed in plants, which have enzymes that can combine glucose (a) and fructose (b). Plants that do this include sugar cane and sugar beets.
When we eat sucrose, enzymes in our digestive system break the bond that holds the glucose and the fructose together. That releases the two monomers, which can then be absorbed into our cells and used for energy.
Another disaccharide that you might have heard about is lactose.
Milk: nutrition facts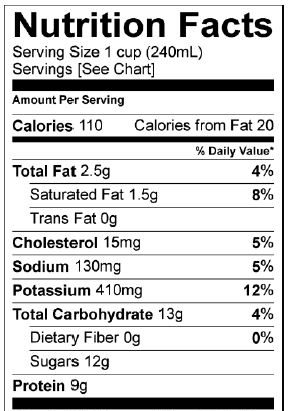 |
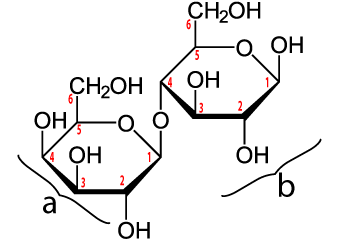 |
Lactose is milk sugar. Notice how in the nutrition facts for a cup of milk, there’s a listing for 12 grams of sugar. That sugar is lactose. Lactose is composed of two linked monosaccharides: galactose (“a” in the diagram above) and glucose (at “b”).
The first food that mammals eat after being born is milk. To digest milk, infant mammals (everything from baby bats to baby whales) produce an enzyme called lactase that breaks lactose apart, making both glucose and galactose available to the cells of the body.
When mammals are weaned, they stop drinking milk, and switch to eating whatever food their species eats. Since it would be wasteful for the body to produce lactose-digesting enzymes when milk is no longer part of an individual’s diet, there’s natural selection for most mammals to turn off the genes for lactase production after weaning. As a result, most adult mammals are lactose intolerant.
That’s true of humans, too. What that means is that for many people, ingesting a food with lactose leads to indigestion, with symptoms like gas and diarrhea. But why are some people lactose intolerant, while other’s aren’t? If you’re interested, you can read more about lactose intolerance here. And if you want to learn about the evolution of lactose tolerance in certain human populations, you can watch this video.
Both monosaccharides and disaccharides are classified sugars. Why? Because they’re both sweet. Sugars —both monosaccharides and disaccharides — have a shape that lets them fit into certain taste receptors on the surface of our tongues. Those receptors send messages to the brain, which gives us a sensation of sweetness.
We’ll see below that that’s not true of other carbohydrates. But before moving on to other carbohydrates, let’s consolidate what we’ve learned with a quiz.
2. Sugars: Checking Understanding
[qwiz qrecord_id=”sciencemusicvideosMeister1961-sugars (checking understanding: HS)”]
[h]Sugars
[i]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem2_Monosaccharides|6e29ecb2bae76″ question_number=”1″] Monosaccharides are the monomers of[hangman]
[c]IGNhcmJvaHlkcmF0ZXM=[Qq]
[f]IENvcnJlY3Qh[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem2_Monosaccharides|6e248a573ba76″ question_number=”2″] The ratio of hydrogen to oxygen in a monosaccharide is always [hangman] to one.
[c]IHR3bw==[Qq]
[f]IEdyZWF0IQ==[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem2_Monosaccharides|6e1e92f8c3676″ question_number=”3″] The “hydrate” part of the word “carbohydrate” refers to[hangman]
[c]IHdhdGVy[Qq]
[f]IEdyZWF0IQ==[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem2_Monosaccharides|6e01532363276″ question_number=”9″] This sugar circulates in the bloodstream. Inability to control this sugar is associated with diabetes.
This sugar is called [hangman]
[c]IGdsdWNvc2U=[Qq]
[f]IEdyZWF0IQ==[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem2_Monosaccharides|6dfbcb8725a76″ question_number=”10″] A monosaccharide used as a sweetener in many soft drinks.
[c]IHN1Y3Jvc2U=[Qq]
[f]IE5vLiBXaGlsZSBzdWNyb3NlIGlzIHVzZWQgYXMgYSBzd2VldGVuZXIsIGl0JiM4MjE3O3Mgbm90IGEgbW9ub3NhY2NoYXJpZGUu[Qq]
[c]IGdsdWNvc2U=[Qq]
[f]IE5vLiBHbHVjb3NlIGlzIGEgbW9ub3NhY2NoYXJpZGUsIGJ1dCBpdCYjODIxNztzIG5vIGFzIGNvbW1vbmx5IHVzZWQgYXMgYSBzb2Z0LWRyaW5rIHN3ZWV0ZW5lciBhcyBhbm90aGVyIG9uZSBvZiB0aGVzZSBjaG9pY2VzLg==[Qq]
[c]IGZydW N0b3Nl[Qq]
[f]IEV4Y2VsbGVudC4gRnJ1Y3Rvc2UgaXMgYSBtb25vc2FjY2hhcmlkZSBmcmVxdWVudGx5IHVzZWQgYXMgYSBzd2VldGVuZXIgaW4gc29mdCBkcmlua3Mu
Cg==[Qq]
[q] Table sugar is a disaccharide. This disaccharide also known as [hangman].
[c]IHN1Y3Jvc2U=
Cg==[Qq]
[q] The disaccharide in milk is [hangman]
[c]bGFjdG9zZQ==
Cg==[Qq]
[q]Monosaccharides get combined into disaccharides through [hangman] synthesis (shown below)
[c]ZGVoeWRyYXRpb24=
Cg==[Qq]
[q]Bloating, flatulence, and diarrhea associated with drinking milk are symptoms of possible [hangman] intolerance
[c]bGFjdG9zZQ==
Cg==[Qq]
[q]Most mammals can only produce lactose-digesting [hangman] until they are weaned. After that, lactose can no longer be [hangman].
[c]ZW56eW1lcw==[Qq]
[c]ZGlnZXN0ZWQ=[Qq]
[/qwiz]
3. Polysaccharides
In the section above, we saw how some enzymes stitch two monosaccharides together to create disaccharides. There are other enzymes that don’t stop at two, but keep on connecting monosaccharides into much longer chains. These chains are called polysaccharides. We’ll examine three: starch, glycogen, and cellulose.
3a.Starch
Starch is a polymer of glucose, consisting of dozens or hundreds of glucose molecules that are chained together.
Starch is used by plants for long term energy storage.

Think about a potato. The leaves of plants perform photosynthesis, which creates glucose.
Glucose is a high-energy molecule, perfect for powering cellular activity. But glucose is not well-suited for long-term energy storage. To store chemical energy, cells in the leaves of a potato plant convert glucose into the disaccharide sucrose, which gets transported to an underground part of the stem (called a “tuber”). This tuber is what we call a potato, and in the cells of the potato, the sucrose gets converted to starch.
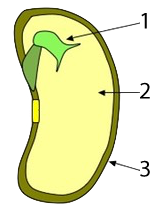
In seeds, such as rice, wheat, and corn, much of the mass of the seed consists of starchy tissue (“2”), the function of which is to sustain the seed’s embryo (“1,” at right) until it sprouts and can start to create its own food through photosynthesis (“3” is a protective coat that surrounds and protects the seed).
 The enzymes in our intestines have no difficulty breaking the bonds that hold the glucose monomers together in starch. That means that starch can easily be converted into simpler carbohydrates, including glucose. Consequently, while starchy foods evolved to store energy for plants, they are also sources of abundant energy for humans (and many other animals).
The enzymes in our intestines have no difficulty breaking the bonds that hold the glucose monomers together in starch. That means that starch can easily be converted into simpler carbohydrates, including glucose. Consequently, while starchy foods evolved to store energy for plants, they are also sources of abundant energy for humans (and many other animals).
You can see evidence of the energy-rich nature of starch to your right, in the nutrition label for a small, 148 gram potato. Most of the potato is water. Of what’s left, notice that the label lists 26 grams of “Total Carbohydrate.” Eat twenty small potatoes like that, and it’s about all the calories you need. That’s why potatoes were the star of one of my all-time favorite science fiction novels and movies, The Martian, by Andy Weir.
On a more down to Earth level, the energy-rich nature of starch is why tubers like potatoes and sweet potatoes, along with starchy seeds (wheat, corn, and rice), are the staple foods upon which much of modern civilization depends.
3b. Glycogen
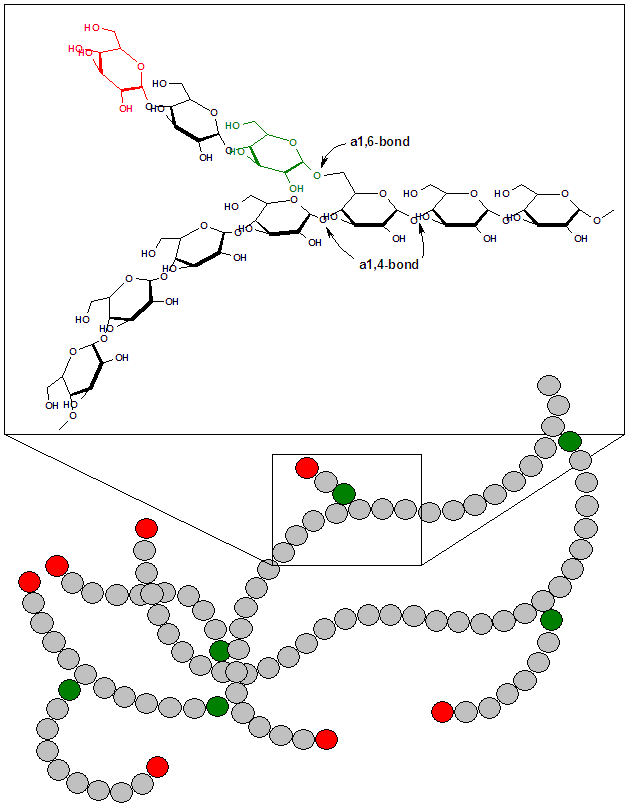 Glycogen is a starch-like molecule that’s stored in our livers and muscle tissue. In fact, it’s so similar to starch that it’s sometimes called “animal starch.” The only difference is that it’s slightly more branched.
Glycogen is a starch-like molecule that’s stored in our livers and muscle tissue. In fact, it’s so similar to starch that it’s sometimes called “animal starch.” The only difference is that it’s slightly more branched.
Glycogen is used by humans and other animals for short-term energy storage. After you eat a starchy meal, your digestive system takes the starch, and, in several steps, converts it into glucose. After being absorbed into the cells lining your intestines, the glucose diffuses into your bloodstream. While some of that glucose might be used right away by cells in your muscles, brain, and other organs, your body can also temporarily store the energy in the glucose by reconverting it into glycogen.
At any moment, a small percentage of your body weight consists of glycogen: about a kilogram or two of glycogen (about 4 pounds) gets stored in liver and muscle tissues of the body of a person my size (about 85 kilograms, or 187 pounds). What’s that glycogen doing? It’s waiting for your body to need carbohydrate. When it does, the cells in your liver and muscle tissue will receive chemical instructions telling them to break down the glycogen into glucose, which can be used by muscles and other organs.
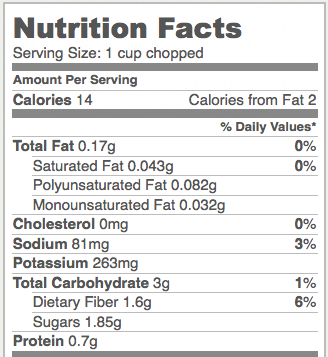
3c. Cellulose
Cellulose is another polysaccharide built from glucose monomers. It’s the most abundant organic polymer on Earth. However, unlike the energy storage polysaccharides like starch and glycogen, cellulose’s function is structural. It’s the primary component of plant cell walls, and it’s commonly referred to as “plant fiber.” Cotton is about 90% cellulose. Wood is about 50% cellulose (wikipedia).
|
Celery
|
|
Celery is a food that’s very high in cellulose. Here’s a nutrition label for one cup of chopped celery. Notice the 1.6 grams of dietary fiber. That’s cellulose.
Notice that celery is a very low calorie food, with only 14 food calories in per cup. By contrast, a cup of corn kernels, which is mostly starch, has over 10 times as many calories (164). If both starch and cellulose are glucose polymers, why do foods with starch have so many more calories/gram than foods with cellulose?
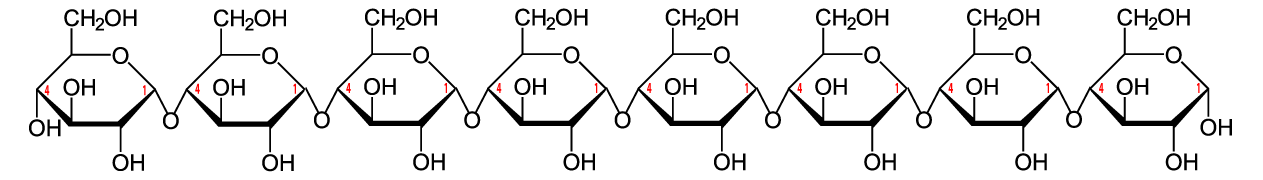
Here’s the structural formula for cellulose. The first thing to notice is that cellulose is a polymer of glucose.
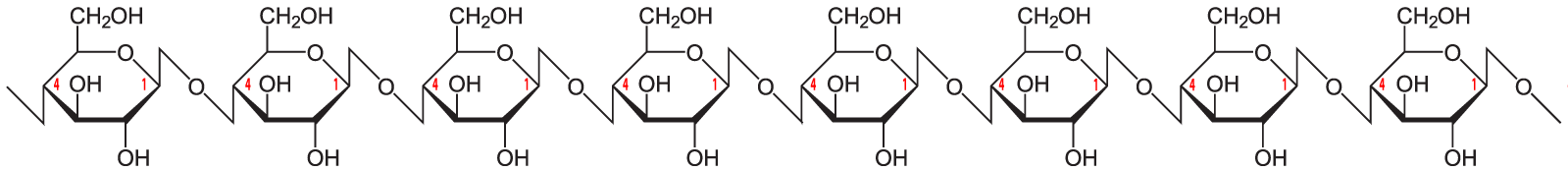
Now look at a section of cellulose on the left and starch on the right.
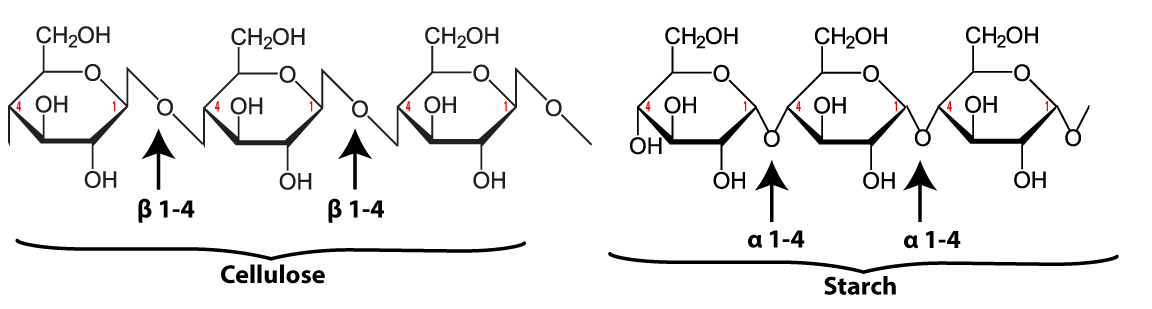
The biochemistry is a bit beyond what’s typically covered in an introductory high school course. The main thing to note is that the bond between the glucose monomers in cellulose is different from the bond between the glucose monomers in starch. While we have enzymes that can break the bond in starch (called an “α 1-4 bond), we don’t have enzymes that can break down the bond between adjacent glucose monomers in cellulose (called a β 1-4 bond).
As a result, when you ingest foods high in cellulose, you don’t digest the cellulose into glucose. Consequently, high fiber foods (leaves such as lettuce or spinach), or bran (which is from the hard outer parts of grains) are relatively low in calories. But while fiber doesn’t provide you with calories, it is an important part of the diet. You need fiber to move food through your digestive system (and avoid constipation). Many studies indicate that diets high in fiber provide protection against various forms of cancer, particularly cancer of the colon (the large intestine).(Johns Hopkins)
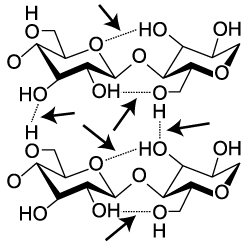 Cellulose is not only hard to digest. It’s also strong. Think about materials like cotton fiber, or wood. Part of their strength comes from interactions between cellulose molecules. When arrayed side by side, cellulose strands form hydrogen bonds with one another. In the diagram to the left, these hydrogen bonds are indicated by dashed lines between one glucose monomer and the next. While hydrogen bonds are weaker than covalent bonds, the collective impact of the bonds between adjacent cellulose strands give cellulose significant strength.
Cellulose is not only hard to digest. It’s also strong. Think about materials like cotton fiber, or wood. Part of their strength comes from interactions between cellulose molecules. When arrayed side by side, cellulose strands form hydrogen bonds with one another. In the diagram to the left, these hydrogen bonds are indicated by dashed lines between one glucose monomer and the next. While hydrogen bonds are weaker than covalent bonds, the collective impact of the bonds between adjacent cellulose strands give cellulose significant strength.
All vertebrates (animals with backbones) lack the genes that would enable us to produce enzymes to break cellulose down to glucose monomers. However, several mammalian species have formed mutualistic (win-win) partnerships with bacteria that can digest cellulose. These species are called ruminants, a group that includes cows, goats, deer, camels, and sheep. In the insect world, termites have evolved a parallel relationship with a single celled protist that can also digest cellulose.
 |
 |
| Gazelles and Termites: two examples of organisms that, with the help of mutualistic partners, can digest cellulose (images from wikipedia) | |
10. Carbohydrates: Checking Understanding
Let’s end with a quiz about polysaccharides. However, just to help all of these concepts stick in memory, I’ll throw in a couple of questions about other carbohydrate topics.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-carbs, checking understanding (HS)”] [h]
Polysaccharides (and carbohydrates in general)
[i]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c5f07c15fb15″ question_number=”1″] A simple sugar (such as glucose) is a [hangman]
[c]IG1vbm9zYWNjaGFyaWRl[Qq]
[f]IEdvb2Qh[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c52840176315″ question_number=”2″] A sugar composed of two monosaccharides is a[hangman]
[c]IGRpc2FjY2hhcmlkZQ==[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c4c67623fb15″ question_number=”3″] Carbohydrates like cellulose and starch are classified as[hangman]
[c]IHBvbHlzYWNjaGFyaWRlcw==[Qq]
[f]IEdvb2Qh[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c45b5c010315″ question_number=”4″] Of the following polysaccharides, the one that humans can’t digest for food energy is
[c]IGdseWNvZ2Vu[Qq]
[f]IE5vLiBHbHljb2dlbiBpcyBzb21ldGltZXMgY2FsbGVkICYjODIyMDthbmltYWwgc3RhcmNoLiYjODIyMTsgSWYgd291bGQgYmUgdW51c3VhbCB0byBlYXQgYSBsb3Qgb2YgaXQsIGJ1dCBpZiB5b3UgaW5nZXN0ZWQgaXQsIHlvdSBjb3VsZCBkZWZpbml0ZWx5IGJyZWFrIGl0cyBib25kcyBhcGFydCB0byBwcm9kdWNlIGdsdWNvc2UuIFRoZSBwb2x5c2FjY2hhcmlkZSB5b3UmIzgyMTc7cmUgbG9va2luZyBmb3IgaXMgdGhlIG9uZSB0aGF0IG1ha2VzIHVwIHBsYW50IGNlbGwgd2FsbHMu[Qq]
[c]IGNlbGx1 bG9zZQ==[Qq]
[f]IENvcnJlY3QuIFRoZSBib25kcyBob2xkaW5nIGdsdWNvc2UgbW9ub21lcnMgdG9nZXRoZXIgaW4gY2VsbHVsb3NlIGNhbiYjODIxNzt0IGJlIGJyb2tlbiBkb3duIGJ5IHRoZSBodW1hbiBkaWdlc3RpdmUgc3lzdGVtLiBBcyBhIHJlc3VsdCwgY2VsbHVsb3NlIGlzIG5vdCBhIHNvdXJjZSBvZiBmb29kIGVuZXJneS4=[Qq]
[c]IHN0YXJjaA==[Qq]
[f]IE5vLiBTdGFyY2ggaXMgYSBwb2x5c2FjY2hhcmlkZSB0aGF0IGh1bWFucyBjYW4gZWFzaWx5IGJyZWFrIGRvd24gdG8gZ2x1Y29zZSBtb25vbWVycyB0aGF0IGNhbiBzZXJ2ZSBhcyBhbiBlbmVyZ3kgc291cmNlLsKgVGhlIHBvbHlzYWNjaGFyaWRlIHlvdSYjODIxNztyZSBsb29raW5nIGZvciBpcyB0aGUgb25lIHRoYXQgbWFrZXMgdXAgcGxhbnQgY2VsbCB3YWxscy4=
Cg==[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c3f73e01b715″ question_number=”5″] The polysaccharide that animals make to store away the excess glucose that they’ve eaten is
[c]IGdseW NvZ2Vu[Qq]
[f]IFllcy4gR2x5Y29nZW4gaXMgc29tZXRpbWVzIGNhbGxlZCAmIzgyMjA7YW5pbWFsIHN0YXJjaC4mIzgyMjE7IFdoZW4geW91IHRha2UgaW4gdG9vIG11Y2ggZ2x1Y29zZSwgeW91ciBib2R5IHdpbGwgY29udmVydCBpdCB0byBnbHljb2dlbiwgYW5kIHN0b3JlIGl0IGF3YXkgaW4geW91ciBsaXZlciBhbmQgbXVzY2xlIHRpc3N1ZS4=[Qq]
[c]IGNlbGx1bG9zZQ==[Qq]
[f]IE5vLiBDZWxsdWxvc2UgaXMgYSBwb2x5c2FjY2hhcmlkZSBtYWRlIGJ5IHBsYW50cy4gSXQmIzgyMTc7cyBhbHNvIGNhbGxlZCBwbGFudCBmaWJlci4gSGVyZSYjODIxNztzIGEgaGludDogdGhpcyBwb2x5c2FjY2hhcmlkZSBpcyBzb21ldGltZXMgY2FsbGVkICYjODIyMDthbmltYWwgc3RhcmNoLiYjODIyMTs=[Qq]
[c]IHN0YXJjaA==[Qq]
[f]IE5vLiBTdGFyY2ggaXMgYSBwb2x5c2FjY2hhcmlkZSBtYWRlIGJ5IHBsYW50cyB0byBzdG9yZSBleGNlc3MgZ2x1Y29zZS4gWW91JiM4MjE3O3JlIGxvb2tpbmcgZm9yIGEgcG9seXNhY2NoYXJpZGUgdGhhdCYjODIxNztzIG1hZGUgYnkgYW5pbWFscy4=[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c2db30570f15″ question_number=”8″] Which of the foods below would consist of the highest amount of starch?
[c]IGFuIGFwcGxl[Qq]
[f]IE5vLiBMaWtlIG1vc3QgZnJ1aXRzLCBhcHBsZXMgaGF2ZSBhIGxvdCBvZiBzdWdhciAocHJpbWFyaWx5IGZydWN0b3NlKSBhbmQgYSBsb3Qgb2YgZmliZXIgKGZyb20gcGxhbnQgY2VsbCB3YWxscyku[Qq]
[c]IEJsYWNr IGJlYW5z[Qq]
[f]IEV4Y2VsbGVudC4gQmVhbnMgYXJlIHNlZWRzLCBhbmQgbGlrZSBtb3N0IHNlZWRzLCB0aGV5IGhhdmUgYSBsb3Qgb2Ygc3RvcmVkIGVuZXJneSB0byBzdXBwb3J0IHRoZSBzZWVkcyBlbWJyeW8uIFRoYXQgZW5lcmd5IGlzIGluIHRoZSBmb3JtIG9mIHN0YXJjaC4=[Qq]
[c]IExldHR1Y2U=[Qq]
[f]IE5vLiBMZXR0dWNlIGhhcyBhIGxvdCBvZiBmaWJlciwgYnV0IGhhcmRseSBhbnkgc3RhcmNoLg==
Cg==[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c282b6933715″ question_number=”9″] When you eat foods like spaghetti, your body will take the starch in the spaghetti and, in several steps, convert the spaghetti into its monomer, which is [hangman].
[c]IGdsdWNvc2U=[Qq]
[f]IEdyZWF0IQ==[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c1cac6e7db15″ question_number=”11″] The hydrogen bonds indicated by the arrows would be found in [hangman].
[c]IGNlbGx1bG9zZQ==[Qq]
[f]IEdvb2Qh[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c16ff9181f15″ question_number=”12″] If your doctor tells you to get more fiber in your diet, then she or he is telling you to ingest which kind of carbohydrate?
[c]IGdseWNvZ2Vu[Qq]
[f]IE5vLiBHbHljb2dlbiBpcyBhIG1vbGVjdWxlIHRoYXQmIzgyMTc7cyB2ZXJ5IHNpbWlsYXIgdG8gc3RhcmNoLiBJdCBzdG9yZXMgZW5lcmd5LCBidXQgaXQmIzgyMTc7cyBub3QgZmliZXIuIEhlcmUmIzgyMTc7cyBhIGhpbnQ6IHRoZXJlJiM4MjE3O3MgYSBsb3Qgb2YgZmliZXIgaW4gcGxhbnQgY2VsbCB3YWxscy4gV2hhdCBtb2xlY3VsZSBtYWtlcyB1cCB0aG9zZSBjZWxsIHdhbGxzPw==[Qq]
[c]IGNlbGx1 bG9zZQ==[Qq]
[f]IFllcy4gQ2VsbHVsb3NlIGlzIHRoZSBtb2xlY3VsZSB0aGF0IG1ha2VzIHVwIHBsYW50IGZpYmVyLg==[Qq]
[c]IFN0YXJjaA==[Qq]
[f]IE5vLlN0YXJjaCBpcyBhIHBvbHlzYWNjaGFyaWRlIHRoYXQgc3RvcmVzIGVuZXJneSwgYnV0IGl0JiM4MjE3O3Mgbm90IGZpYmVyLiBIZXJlJiM4MjE3O3MgYSBoaW50OiB0aGVyZSYjODIxNztzIGEgbG90IG9mIGZpYmVyIGluIHBsYW50IGNlbGwgd2FsbHMuIFdoYXQgbW9sZWN1bGUgbWFrZXMgdXAgdGhvc2UgY2VsbCB3YWxscz8=[Qq]
[c]IExhY3Rvc2U=[Qq]
[f]IE5vLiBMYWN0b3NlIGlzIGEgZGlzYWNjaGFyaWRlLCBhbmQgbm90IGEgc291cmNlIG9mIGZpYmVyLsKgSGVyZSYjODIxNztzIGEgaGludDogdGhlcmUmIzgyMTc7cyBhIGxvdCBvZiBmaWJlciBpbiBwbGFudCBjZWxsIHdhbGxzLiBXaGF0IG1vbGVjdWxlIG1ha2VzIHVwIHRob3NlIGNlbGwgd2FsbHM/
Cg==[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c0f492a1eb15″ question_number=”13″] The polysaccharide that plants make to store away chemical energy from the excess glucose that they’ve made during photosynthesis is
[c]IGdseWNvZ2Vu[Qq]
[f]IE5vLiBHbHljb2dlbiBpcyBzb21ldGltZXMgY2FsbGVkICYjODIyMDthbmltYWwgc3RhcmNoLiYjODIyMTsgV2hlbiB5b3UgdGFrZSBpbiB0b28gbXVjaCBnbHVjb3NlLCB5b3VyIGJvZHkgd2lsbCBjb252ZXJ0IGl0IHRvIGdseWNvZ2VuLCBhbmQgc3RvcmUgaXQgYXdheSBpbiB5b3VyIGxpdmVyIGFuZCBtdXNjbGUgdGlzc3VlLg==[Qq]
[c]IGNlbGx1bG9zZQ==[Qq]
[f]IE5vLiBDZWxsdWxvc2UgaXMgYSBwb2x5c2FjY2hhcmlkZSBtYWRlIGJ5IHBsYW50cy4gSXQmIzgyMTc7cyBhbHNvIGNhbGxlZCBwbGFudCBmaWJlci4gSXQmIzgyMTc7cyBhbiBlc3NlbnRpYWwgcGFydCBvZiBjZWxsIHdhbGxzLCBidXQgaXQmIzgyMTc7cyBub3QgdXNlZCB0byBzdG9yZSBlbmVyZ3ku[Qq]
[c]IHN0YX JjaA==[Qq]
[f]IFllcy4gU3RhcmNoIGlzIGEgcG9seXNhY2NoYXJpZGUgbWFkZSBieSBwbGFudHMgdG8gc3RvcmUgdGhlIGNoZW1pY2FsIGVuZXJneSBpbiBnbHVjb3NlLg==
Cg==[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem4_carbohydrates_cumulative|7c0439f1a3b15″ question_number=”14″] If the molecule below is starch, then the monomers making up this molecular are [hangman].
[c]IGdsdWNvc2U=[Qq]
[f]IEdvb2Qh[Qq]
[/qwiz]
Links
- Lipids (next tutorial in this series)
- Molecules of Life (HS) Main Menu