Link to Chemistry for Biology Students: Study Guide/Student Worksheet
1. Introduction
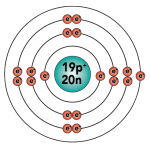
In the last tutorial, we learned about the Octet Rule and saw how it can be used to show which energy levels the electrons in an atom will inhabit. You can see the Octet Rule in action in this diagram of potassium.
In the living systems that you’ll study in biology, atoms are almost always bonded to other atoms. That’s the topic of this and the next two tutorials: chemical bonding.
2. Learning the symbols for the key elements in living things: C, H, N, O, P, S, Ca, K
Living things are mostly made of the following elements: carbon, hydrogen, nitrogen, oxygen, phosphorus, sulfur, calcium, and potassium. Because these elements are so common, it’s useful to know their symbols by heart.
Start by studying this list:
- C: Carbon
- H: Hydrogen
- N: Nitrogen
- O: Oxygen
- P: Phosphorus
- S: Sulfur
- Ca: Calcium
- K: Potassium
If you combine the symbols to make the following nonsense word, CHNOPSCaK, you’ll be able to recite these elements from memory. It’ll also be worth your while to practice using these flashcards.
[qdeck style=”border: 2px solid black; ” random = “true” qrecord_id=”sciencemusicvideosMeister1961-Element Symbols to Know Flashcards (M3)”]
[h] Flashcards: Element Symbols to know
[i] If you haven’t used a set of flashcards on Learn-Biology.com before, here’s how they work.
- Click ‘Flip’ to see the answer to each card.
- If you know it, click ‘Got it.”
- If you don’t know it as well as you’d like, click ‘Need more practice,’ and that card will go to the bottom of the deck so you can practice it again.
- ‘Shuffle’ lets you shuffle the deck.
[start]
[q] What’s the chemical symbol for carbon?
[textentry]
[a] The symbol for carbon is C
[q] What’s the chemical symbol for hydrogen?
[textentry]
[a] The symbol for hydrogen is H
[q] What’s the chemical symbol for nitrogen?
[textentry]
[a] The symbol for nitrogen is N
[q] What’s the chemical symbol for oxygen?
[textentry]
[a] The chemical symbol for oxygen is O
[q] What’s the chemical symbol for phosphorus?
[textentry]
[a] The symbol for phosphorus is P
[q] What’s the chemical symbol for sulfur?
[textentry]
[a] The symbol for sulfur is S
[q] What’s the chemical symbol for calcium?
[textentry]
[a] The symbol for calcium is Ca
[q] What’s the chemical symbol for potassium
[textentry]
[a] The symbol for potassium is K
[q] What’s C the symbol for?
[textentry]
[a] C is the symbol for carbon.
[q] What’s H the symbol for?
[textentry]
[a] H is the symbol for hydrogen.
[q] What’s N the symbol for?
[textentry]
[a] N is the symbol for nitrogen.
[q] What’s O the symbol for?
[textentry]
[a] O is the symbol for oxygen.
[q] What’s P the symbol for?
[textentry]
[a] P is the symbol for phosphorus.
[q] What’s S the symbol for?
[textentry]
[a] S is the symbol for sulfur.
[q] What’s Ca the symbol for?
[textentry]
[a] Ca is the symbol for calcium.
[q] What’s K the symbol for?
[textentry]
[a] K is the symbol for potassium.
[x]
If you want more practice, please press the restart button below. Otherwise, continue reading below.
[restart]
[/qdeck]
3. Interactive Reading: Compounds, Molecules, and Formulas
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Compounds, Molecules, Formulas IR (M3)”]
[h]Interactive Reading: Compounds, Molecules, and Formulas
[i]The following reading includes fill-in-the-blanks, true-false, and multiple choice questions. Remember that reading in a way where you interact with the text improves your ability to remember what you’ve read.
[q labels = “top”]
When elements bind together through chemical bonding, they form compounds (a substance composed of two or more __________ that are chemically bonded together). The smallest piece of a compound that still has the ___________ of that compound is a molecule.
What does that mean? Water is a compound. Imagine that you had a spoonful of water. Take that spoonful, and pour off half. Then take what’s left, and pour off half again. Repeat…billions of times. Eventually, you’d wind up with a single _________ of water. It’s the smallest amount of water that is still water.
At that point, if you chemically broke that water ___________ apart (by running electricity through it, for example), you would be separating the hydrogen atoms from the oxygen atom. The result would be molecules of hydrogen and oxygen…which, of course, is not _________.
[l]elements
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]molecule
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]properties
[fx] No. Please try again.
[f*] Great!
[l]water
[fx] No. Please try again.
[f*] Excellent!
[q] A compound has a chemical formula. You can think of the formula in two ways.
- A formula shows the number of atoms of each _______ that make up a molecule. For example, because a water molecule consists of two hydrogen atoms and one oxygen atom, the ___________ for water is H2O.
- The formula is a recipe. It says that if you wanted to make water from hydrogen gas and oxygen gas, you’d need two parts __________ to one part ________.
[l]oxygen
[fx] No. Please try again.
[f*] Great!
[l]element
[fx] No. Please try again.
[f*] Good!
[l]hydrogen
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]formula
[fx] No. Please try again.
[f*] Excellent!
[q]Interpreting formulas is easy. The symbols represent the elements that make up that compound. If there’s a number after the symbol, that indicates the number of atoms. If there’s not a number, there’s just one atom.
Some of the compounds that make up living things are similar to water and methane: they’re relatively small and simple. Others, like hemoglobin, the protein that carries oxygen in your bloodstream, are much larger. Hemoglobin’s chemical formula is C2952H4664O832N812S8Fe4.
Just to make sure you’ve got this:
Glucose is a very basic form of sugar. Its formula is C6H12O6. How many oxygen atoms are in glucose?
[c]MA==[Qq]
[c]MTI=[Qq]
[c]Ng ==[Qq]
[f]Tm8uIExvb2sgYXQgdGhlIHNtYWxsIG51bWJlciAodGhlIHN1YnNjcmlwdCkgdGhhdCBmb2xsb3dzICYjODIyMDtPLiYjODIyMTs=[Qq]
[f]Tm8uIFRoZXJlIGFyZSAxMiBoeWRyb2dlbiBhdG9tcy4gTG9vayBhdCB0aGUgc21hbGwgbnVtYmVyICh0aGUgc3Vic2NyaXB0KSB0aGF0IGZvbGxvd3MgJiM4MjIwO08uJiM4MjIxOw==[Qq]
[f]Q29ycmVjdC4gVGhlcmUgYXJlIDYgb3h5Z2VuIGF0b21zIGluIGdsdWNvc2Uu[Qq]
[q]Glucose’s chemical formula is C6H12O6. How many hydrogen atoms are in glucose?
[c]MA==[Qq]
[c]MT I=[Qq]
[c]Ng==[Qq]
[f]Tm8u[Qq]
[f]RXhjZWxsZW50LiBUaGVyZSBhcmUgMTIgaHlkcm9nZW4gYXRvbXMgaW4gZ2x1Y29zZS4=[Qq]
[f]Tm8uwqBUaGVyZSBhcmUgNiBveHlnZW4gYXRvbXMgaW4gZ2x1Y29zZSwgYXMgd2VsbCBhcyA2IG94eWdlbiBhdG9tcy4gVG8gZmluZCB0aGUgbnVtYmVyIG9mIGh5ZHJvZ2VuIGF0b21zLMKgbG9vayBhdCB0aGUgc21hbGwgbnVtYmVyICh0aGUgc3Vic2NyaXB0KSB0aGF0IGZvbGxvd3MgJiM4MjIwO0guJiM4MjIxOw==[Qq]
[x]
[restart]
[/qwiz]
4. Compounds, Molecules, Formulas Flashcards
Just to make sure that you’ve mastered some of the chemical symbols that you practiced above, and the terms I’ve just introduced, go through these flashcards. You’ll also see a few questions from the previous tutorials in this series, just to make sure that they stay in your memory.
[qdeck style=”border: 2px solid black;” qrecord_id=”sciencemusicvideosMeister1961-Compounds, Molecules, Formulas Flashcards (M3)”][h] Flashcards: Compounds, Molecules, and Formulas
[i] If you haven’t used a set of flashcards on sciencemusicvideos before, here’s what you need to know.
- Click ‘Flip’ to see the answer to each card.
- If you know it, click ‘Got it.”
- If you don’t know it as well as you’d like, click ‘Need more practice,’ and that card will go to the bottom of the deck so you can practice it again.
- ‘Shuffle’ lets you shuffle the deck.
[start]
[q] A substance composed of two or more elements that are chemically bonded together
[textentry]
[a] A substance composed of two or more elements that are chemically bonded together is a compound
[q] The smallest piece of a compound that still has that compound’s chemical properties is a
[textentry]
[a] The smallest piece of a compound that still has that compound’s physical and chemical properties is a molecule
[q] CH4, H2O, NH3, C6H12O6, and all such combinations of chemical symbols and numbers showing the number of atoms of each element in a molecule are called chemical ________
[textentry]
[a] These combinations of chemical symbols and numbers that show the number of atoms of each element that make up a molecule are called chemical formulas
[q] In the compound C6H12O6, how many hydrogen atoms are there?
[textentry]
[a] In the compound C6H12O6, there are twelve hydrogen atoms.
[q] In the compound C6H12O6, how many carbon atoms are there?
[textentry]
[a] In the compound C6H12O6, there are six carbon atoms
[q] What’s the maximum number of electrons that can fit into the first energy level?
[textentry]
[a] The maximum number of electrons that can fit into the first electron energy level is two.
[q] What’s the maximum number of electrons that can fit into the second energy level?
[textentry]
[a] The maximum number of electrons that can fit into the second energy level is eight.
[q] What’s the maximum number of electrons that can fit into the third energy level?
[textentry]
[a] The maximum number of electrons that can fit into the third energy level is eight.
[q] Fluorine has 9 protons, 10 neutrons, and 9 electrons. Use the Octet rule to draw a fluorine atom
[a] Flourine: the protons and neutrons go into the nucleus. Two electrons go into the first energy level. Seven electrons go into the second energy level.
[x]
If you want more practice, please press the restart button below. Otherwise, follow the links below.
[restart]
[/qdeck]
5. Next steps
If you need more practice, please scroll up to the top and work through this tutorial again. Otherwise, follow the link to Ionic Bonds (the next tutorial in this series).
