Looking for a student learning guide? You’ll find a link on the main menu for your course. Use the “Courses” menu above.
If you’ve already watched the video, click here, or scroll down below the video to start interacting.
Introduction: Ionic Bonds
In the previous tutorials in this series, we learned about the structure of atoms. Now we’ll look at how atoms combine to form compounds and molecules. In this tutorial, we start with one of the major types of chemical bonds: the ionic bond.
1. Atoms are only “happy” when they have complete outer energy levels
1.1. As a biology student, you can get by with a simple model of chemical bonding that works very well to explain what happens when atoms combine to form molecules and compounds. Just pretend that atoms have feelings, and remember these two rules:
-

Argon: with two electrons in its first energy level, eight in the second, and eight in the third, it’s an extremely “happy” atom. Atoms “like” to have their outer shells (or “energy levels”) be full. That means that atoms are “happy” when
- Two electrons are in the first energy level
- Eight electrons are in the second energy level
- Eight electrons are in the third energy level
- If these energy levels aren’t full, then atoms will trade or share electrons so that their outer shells become full.
1.2.Let’s start by looking at a situation when atoms trade electrons. To do this, we’ll examine how table salt forms.

Table salt’s chemical name is sodium chloride, and its formula is NaCl (“Na” is the symbol for sodium, and “Cl” is the symbol for chlorine). Start by solving the challenge below.
[qwiz style = “width:528px; min-height:0px; border: 1px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-ionic bonds, draw sodium”]
[h] 1.3. Apply the Octet Rule to sodium
[q] Sodium (Na) has 11 protons, 12 neutrons, and 11 electrons. Using the Octet rule that we’ve previously learned, draw sodium’s structure, showing all the electrons in their energy levels. Then click below to see if you got it right.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==[x]
[/qwiz]
Make sure you’ve clicked “show the answer” and checked your work before going on.
That outer, lone electron in sodium’s third shell makes it an “unhappy” atom. Sodium can become “happy” by giving up that outer electron. But who will it give it up to?
[qwiz style = “width: 528px; min-height:0px; border: 1px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-ionic bonds, draw chlorine”]
[h] 1.4. Apply the octet rule to chlorine
[q]Chlorine has 17 protons, 18 neutrons, and 17 electrons. Using the Octet rule that we’ve previously learned, draw chlorine’s structure. Then click below to see if you got it right.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==With seven electrons in its outer shell, Chlorine is also an “unhappy” atom, but for the opposite reason compared to sodium. Chlorine needs one additional electron to have a full outer shell.
[x]
[/qwiz]
Make sure you’ve clicked “show the answer” and checked your work before going on.
And just to double-check that you’re getting this, answer the questions below.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-ionic bonds, checking understanding, Na and Cl”]
[h]1.5. Checking Understanding: Sodium and Chlorine
[l]needs to gain an electron
[fx] No. Please try again.
[f*] Excellent!
[l]needs to lose an electron
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[/qwiz]
Sodium and chlorine can make each other happy. The way they do this is through trading electrons. The next section explains how this works. But first, let’s look at a few atoms to make sure that we can distinguish between “happy” and “unhappy ones.”
[qdeck qrecord_id=”sciencemusicvideosMeister1961-Happy, Unhappy Atoms Flashcards (M3)”]
[h]1.6. Flashcards: “Happy” and “Unhappy” Atoms
[i] If you haven’t used a set of flashcards on sciencemusicvideos before, here’s what you need to know.
- Click ‘Flip’ to see the answer to each card.
- If you know it, click ‘Got it.”
- If you don’t know it as well as you’d like, click ‘Need more practice,’ and that card will go to the bottom of the deck so you can practice it again.
- ‘Shuffle’ lets you shuffle the deck.
[!] Card 1+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q] Is neon a “happy” or “unhappy” atom. Briefly explain [textentry]
[a]
Atoms with complete outer energy levels (two electrons in the first energy level, eight in the next two) are happy. Neon is a happy atom.
[!] Card 2+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q] Is lithium a happy or unhappy atom? Explain. [textentry]
[a]
Lithium, because it has one lone electron in its outer energy level, is an unhappy atom.
[!] Card 3+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q]Is fluorine a happy or unhappy atom? Explain.[textentry]
[a]
Fluorine, because it has seven electrons in its outer energy level, is an unhappy atom. It needs one electron to complete its outer shell.
[!] Card 4+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!][!]Card 5 start++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q]Is potassium a happy or unhappy atom? Explain. [textentry]
[a]
Potassium, because it has one electron in its outer energy level, is an unhappy atom. It would need to give away that electron to have a complete outer energy level.
[!] Card 5 end+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[!] Card 6 start++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q] Is argon a “happy” or “unhappy” atom. Briefly explain [textentry]
[a]
Atoms with complete outer electron energy levels (two electrons in the first energy level, eight in the next two) are happy. Argon, because its first three energy levels are full with two, eight, and eight electrons, is a happy atom.
[!] Card 6+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[x]
If you want more practice, please press the restart button below. Otherwise, continue below.
[restart]
[/qdeck]
2. Ionic Bonding Involves Trading Electrons
Introduction: Unhappy atoms (ones that don’t have a complete outer shell of electrons) can achieve happiness by trading electrons with other atoms. As they do, they become charged ions (atoms that have a positive or negative charge). The attraction between positive and negatively charged ions creates a chemical bond called an ionic bond.
Here’s how this works in the formation of table salt (sodium chloride).
2.1. Sodium gives up its outer electron to chlorine.
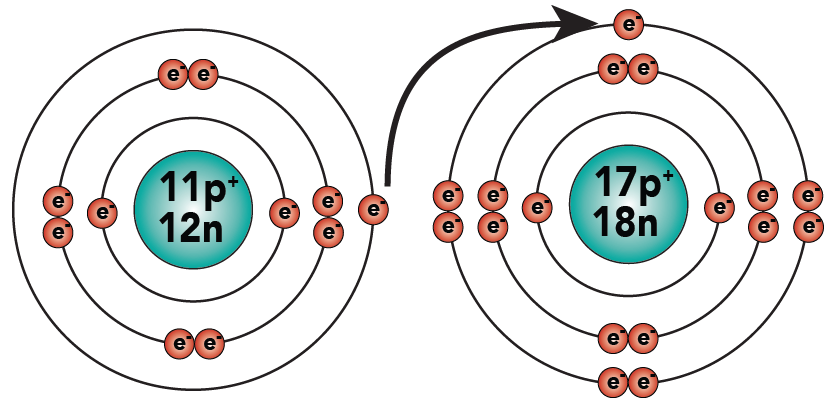
2.2: As a result, both sodium and chlorine have complete outer energy levels. Note that sodium has lost its outermost (third) electron energy level.
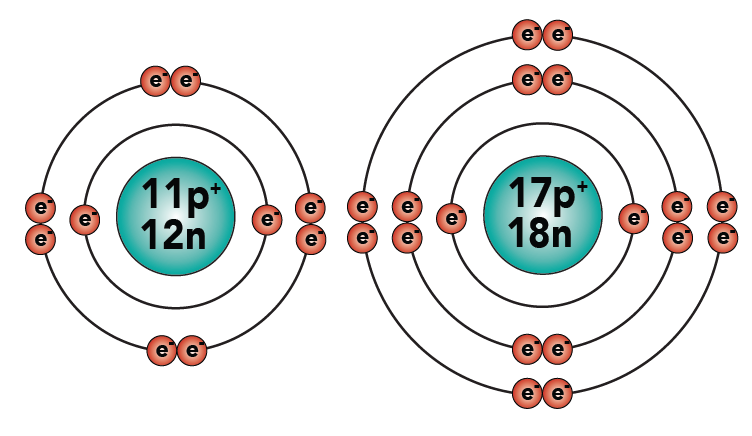
2.3: Sodium’s charge is now +1 because it lost an electron. It now has one more proton (with a positive charge) than electrons (with a negative charge).
Correspondingly, chlorine originally had 17 protons and 17 electrons. Now it has 18 electrons. With an extra electron, it has an overall negative charge of minus 1. To make these charges clear, I’ve added a plus and a minus sign to the diagram. Read the “+” as “plus one.” Read the “-” as “minus one.”
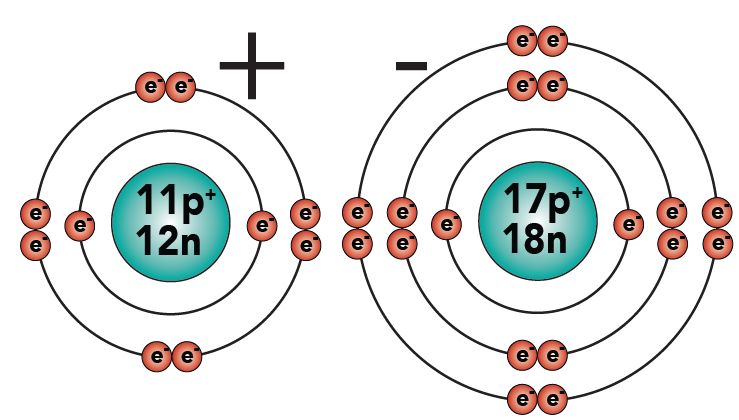
2.4: This difference in charges is the essence of the bond between sodium and chlorine. The expression “opposites attract” is completely true here. The sodium, with its positive charge, will stick to the chlorine, with its negative charge, just like the opposite poles of a magnet attract each other.
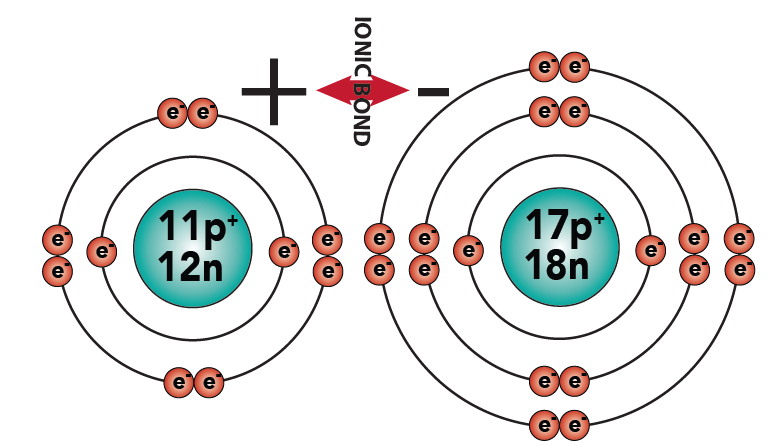
2.5:This type of bond, where atoms trade electrons, creating charged particles that “stick” to one another because of their opposite charges, is called an ionic bond.
2.6: The newly charged atoms are called ions. Sodium has become a positive ion. Chlorine has become a negative ion. And while the system naming ions is beyond the scope of this tutorial, you should note that when chlorine gains an electron and becomes a charged ion, its name changes to “chloride.”
2.7: Atoms connected by ionic bonds form ionic compounds. Study the diagram below to make sure you understand this before proceeding.
2.8: It just takes a bit of imagination to get from two connected ions to the crystals that make up table salt.

A crystal consists of billions of ions regularly arranged in three dimensions. Take a look at the image below.
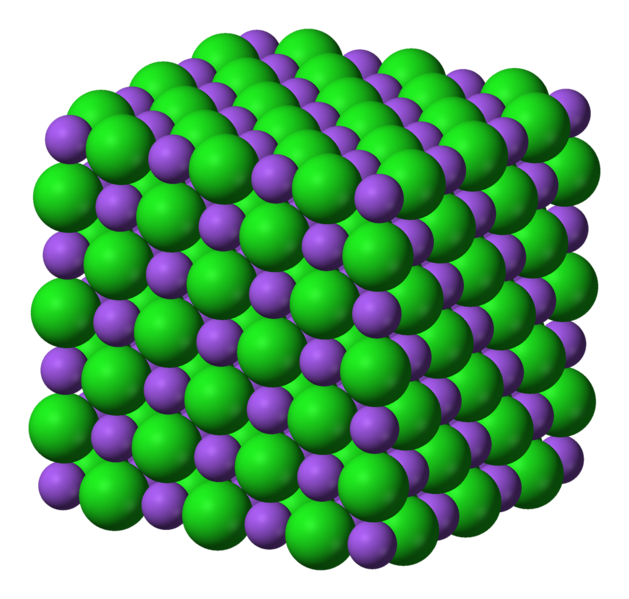
The opposite charges between the positive sodium ions (purple) and the negative chloride ions (green) hold the salt crystal together.
3. A Few More Points about Ionic Bonding
3.1: Atoms will trade one, two, or, less frequently, three electrons fairly readily. But they never trade four, five, six, or seven electrons.
3.2: The charge on an ion is easy to figure out. For every electron that an atom gains, it gains a negative charge. For every electron that an atom loses, it loses a negative charge and gains a positive charge.
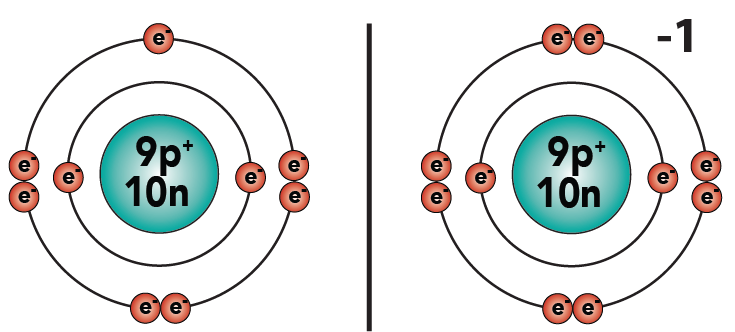
3.3: For example, when fluorine, with seven electrons, gains one more electron to complete its outer shell, it will have a charge of -1. Why? Because it now has one more electron than protons.
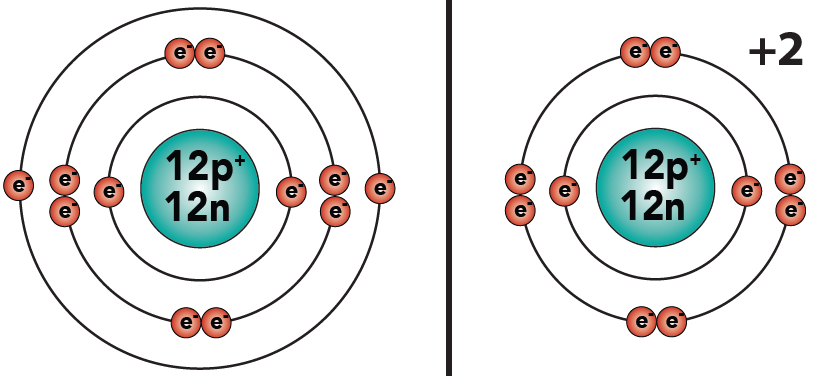
3.4: Similarly, if an atom loses two electrons, it will have a charge of + 2. This is what happens to magnesium (which starts with 12 protons and 12 electrons) when it becomes an ion (with 12 protons and 10 electrons). You can see this in the diagram below
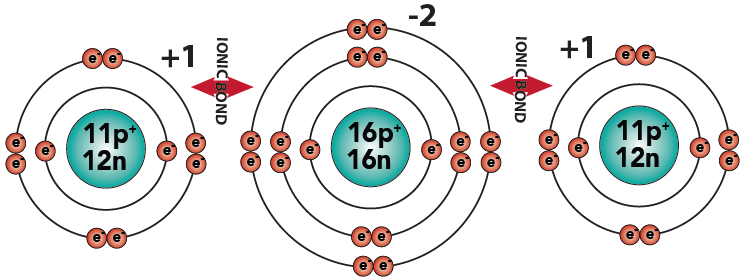
3.5: It’s not always two atoms that make each other “happy” by trading electrons. You can have three atoms that swap electrons in a way so that they’re all happy. This is what happens in the compound sodium sulfide (Na2S). Study the diagram and caption below.
4. Checking Understanding
[qwiz style = “width; 528px; border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Ionic Bonding quiz (M3)”]
[h] Quiz: Ionic Bonding
[i] If you haven’t taken a Learn-Biology quiz before, here’s how it works:
- Each question is multiple choice, but the entire quiz is like a series of flashcards.
- If you get the question right, it comes off the deck. If you get the question wrong, it goes to the bottom of the deck, so you can try it again.
[q] Is this atom a “happy” atom or an “unhappy” atom?
[c]IGhh cHB5[Qq]
[c]IHVuaGFwcHk=[Qq]
[f]IEV4Y2VsbGVudCEgVGhpcyBhdG9tIChOZW9uKSwgaGFzIGEgZnVsbCBvdXRlciBlbmVyZ3kgbGV2ZWwsIHdpdGggZWlnaHQgZWxlY3Ryb25zLCBtYWtpbmcgaXQgYSBoYXBweSBhdG9tLg==
[f]IE5vLiBUaGlzIGF0b20gKE5lb24pLCBoYXMgYSBmdWxsIG91dGVyIGVuZXJneSBsZXZlbCwgd2l0aCBlaWdodCBlbGVjdHJvbnMsIG1ha2luZyBpdCBhIGhhcHB5IGF0b20u[Qq]
[q] Is this atom (Calcium) a “happy” atom or an “unhappy” atom?
Cg==[Qq][c]IFVuaG FwcHk=[Qq]
[f]IE5vLiBUaGlzIGF0b20gKENhbGNpdW0pIGhhcyB0d28gZWxlY3Ryb25zIGluIGl0cyBvdXRlciBzaGVsbC4gQmVjYXVzZSBpdHMgb3V0ZXIgc2hlbGwgaXNuJiM4MjE3O3QgZnVsbCAod2l0aCBlaWdodCBlbGVjdHJvbnMpLCBDYWxjaXVtIGlzIG5vdCAmIzgyMjA7aGFwcHkuJiM4MjIxOw==[Qq]
[f]IE5pY2Ugam9iLiBUaGlzIGF0b20gKENhbGNpdW0pIGhhcyB0d28gZWxlY3Ryb25zIGluIGl0cyBvdXRlciBzaGVsbC4gQmVjYXVzZSBpdHMgb3V0ZXIgc2hlbGwgaXNuJiM4MjE3O3QgZnVsbCAod2l0aCBlaWdodCBlbGVjdHJvbnMpLCBDYWxjaXVtIGlzIG5vdCAmIzgyMjA7aGFwcHkuJiM4MjIxOw==
[q] What’s the easiest pathway to happiness for Calcium?
[c]IFRvIGdpdmUgYXdheSBpdHMgdHdvIGVsZWN0cm9ucyB0 byBhbiBhdG9tIHRoYXQgd2lsbCBhY2NlcHQgdGhlbS4=[Qq]
[c]IFRvIGFjY2VwdCBzaXggZWxlY3Ryb25zIGZyb20gb3RoZXIgYXRvbXM=[Qq]
[f]IFllczogd2l0aCB0d28gZWxlY3Ryb25zIGluIGl0cyBvdXRlcsKgZW5lcmd5IGxldmVsLCB0aGUgbW9zdCBsaWtlbHkgbW92ZSBmb3IgY2FsY2l1bSB3b3VsZCBiZSB0byBnaXZlIHRob3NlIGVsZWN0cm9ucyBhd2F5IHRvIGFub3RoZXIgYXRvbS4=[Qq]
[f]IE5vLiBBbiBhdG9tIHdvdWxkIG5ldmVyIGdpdmUgYXdheSBzaXggZWxlY3Ryb25zLiBXaXRoIHR3byBlbGVjdHJvbnMgaW4gaXRzIG91dGVywqBlbmVyZ3kgbGV2ZWwsIHRoZSBtb3N0IGxpa2VseSBtb3ZlIGZvciBjYWxjaXVtIHdvdWxkIGJlIHRvIGdpdmUgdGhvc2UgZWxlY3Ryb25zIGF3YXkgdG8gYW5vdGhlciBhdG9tLg==
[q] When an atom gives up or accepts electrons to complete its outer electron energy level, it becomes a(n)
[c]IGVsZWN0cmlmaWVkIGF0b20=[Qq]
[c]IHBvc2l0aXZlbHkgY2hhcmdlZCBwYXJ0aWNsZQ==[Qq]
[c]IG5lZ2F0aXZlbHkgY2hhcmdlZCBwYXJ0aWNsZQ==[Qq]
[c]IGlv bg==[Qq]
[f]IE5vLiBUaGVyZSYjODIxNztzIGFub3RoZXIgdGVybSBmb3IgYW4gYXRvbSB0aGF0LCBieSBsb3Npbmcgb3IgZ2FpbmluZyBlbGVjdHJvbnMsIGhhcyBiZWNvbWUgYSBjaGFyZ2VkIHBhcnRpY2xlLg==[Qq]
[f]IE5vLiBJZiBhbiBhdG9tIGxvc2VzIG9uZSBvciBtb3JlIGVsZWN0cm9ucywgaXQgd2lsbCBiZWNvbWUgcG9zaXRpdmVseSBjaGFyZ2VkLiBCdXQgdGhlIHF1ZXN0aW9uIHdhcyBhYm91dCBnYWluaW5nIA==b3I=IGxvc2luZyBlbGVjdHJvbnMuIFdoYXQmIzgyMTc7cyBhIG1vcmUgZ2VuZXJhbCBuYW1lIGZvciBhbiBhdG9tIHRoYXQsIGJ5IGxvc2luZyBvciBnYWluaW5nIGVsZWN0cm9ucywgaGFzIGJlY29tZSBhIGNoYXJnZWQgcGFydGljbGU/[Qq]
[f]IE5vLiBJZiBhbiBhdG9tIGdhaW5zIG9uZSBvciBtb3JlIGVsZWN0cm9ucywgaXQgd2lsbCBiZWNvbWUgcG9zaXRpdmVseSBjaGFyZ2VkLsKgQnV0IHRoZSBxdWVzdGlvbiB3YXMgYWJvdXQgZ2FpbmluZ8Kgb3I=wqBsb3NpbmcgZWxlY3Ryb25zLiBXaGF0JiM4MjE3O3MgYSBtb3JlIGdlbmVyYWwgbmFtZSBmb3LCoGFuIGF0b20gdGhhdCwgYnkgbG9zaW5nIG9yIGdhaW5pbmcgZWxlY3Ryb25zLCBoYXMgYmVjb21lIGEgY2hhcmdlZCBwYXJ0aWNsZT8=[Qq]
[f]IEV4Y2VsbGVudCEgQW4gYXRvbSB0aGF0IGdhaW5zwqBvciBsb3NlcyBlbGVjdHJvbnMgYW5kIGJlY29tZXMgYSBjaGFyZ2VkIHBhcnRpY2xlIGlzIGNhbGxlZCBhbiA=aW9uLg==
Cg==[Qq]
[q] What’s the charge on a calcium ion?
[c]IEl0IGhhcyBubyBjaGFyZ2U=[Qq]
[c]ICsgMQ==[Qq]
[c]ICsg Mg==[Qq]
[c]IC0x[Qq]
[f]IE5vLiBUaGUgZGlhZ3JhbSBzaG93cyBhwqBjYWxjaXVtIGlvbiB3aXRoIDIwIHByb3RvbnMsIGFuZMKgMTggZWxlY3Ryb25zICgyICsgOCArIDggPSAxOCkuwqBUaGUgY2hhcmdlIG9uIHRoZSBpb24gaXMgdGhlIG51bWJlciBvZiBwcm90b25zLCBtaW51cyB0aGUgbnVtYmVyIG9mIGVsZWN0cm9ucy4=[Qq]
[f]IE5vLiBUaGUgZGlhZ3JhbSBpcyBzaG93aW5nIGNhbGNpdW0gd2l0aCAyMCBwcm90b25zLCBidXQgb25seSAxOCBlbGVjdHJvbnMuwqBUaGUgY2hhcmdlIG9uIHRoZSBpb24gaXMgdGhlIG51bWJlciBvZiBwcm90b25zLCBtaW51cyB0aGUgbnVtYmVyIG9mIGVsZWN0cm9ucy4=[Qq]
[f]IEV4Y2VsbGVudC4gVGhlIGRpYWdyYW0gaXMgc2hvd2luZyBjYWxjaXVtIHdpdGggMjAgcHJvdG9ucywgYnV0IG9ubHkgMTggZWxlY3Ryb25zLiBJdHMgY2hhcmdlIGhhcyB0byBiZSArMi4=
[f]IE5vLiBUaGUgZGlhZ3JhbSBpcyBzaG93aW5nIGNhbGNpdW0gd2l0aCAyMCBwcm90b25zLCBidXQgb25seSAxOCBlbGVjdHJvbnMuwqBUaGUgY2hhcmdlIG9uIHRoZSBpb24gaXMgdGhlIG51bWJlciBvZiBwcm90b25zLCBtaW51cyB0aGUgbnVtYmVyIG9mIGVsZWN0cm9ucy4=
Cg==[Qq]
[q] In the diagram shown below, are you looking at an “atom,” an “ion” or a “molecule”?
[c]IEFuIGF0b20=[Qq]
[c]IEFuIG lvbg==[Qq]
[c]IEEgbW9sZWN1bGU=[Qq]
[f]IE5vLiBUaGUgZGlhZ3JhbSBpcyBzaG93aW5nIGZsdW9yaW5lwqB3aXRoIDnCoHByb3RvbnMsIGFuZMKgb25seSAxMMKgZWxlY3Ryb25zLsKgVGhlIGZ1bGwgb3V0ZXIgc2hlbGwgYW5kIHRoZSAtMSBjaGFyZ2UgZ2l2ZSBhIGhpbnQgYXMgdG8gd2hhdCB0aGlzIGlzLg==[Qq]
[f]IFllcy4gVGhlIGRpYWdyYW0gaXMgc2hvd2luZyBmbHVvcmluZcKgd2l0aCA5wqBwcm90b25zLCBhbmQgMTDCoGVsZWN0cm9ucy7CoFRoZSBmdWxsIG91dGVyIHNoZWxsIGFuZCB0aGUgLTEgY2hhcmdlwqBsZXQgeW91IGtub3cgdGhhdCB0aGlzIGZsdW9yaW5lIGhhcyB0cmFkZWQgZWxlY3Ryb25zIHRvIGJlY29tZSBhIGNoYXJnZWQgaW9uLg==
[f]IE5vLiBBIG1vbGVjdWxlIHdvdWxkIGNvbnNpc3Qgb2YgdHdvIG9yIG1vcmUgYXRvbXMgdGhhdCB3ZXJlIGNoZW1pY2FsbHkgYm9uZGVkIHRvIG9uZSBhbm90aGVyLiBUaGUgZnVsbCBvdXRlciBzaGVsbCBhbmQgdGhlIC0xIGNoYXJnZSBnaXZlIGEgaGludCBhcyB0byB3aGF0IHRoaXMgaXMu[Qq]
[x]
[/qwiz]
5. The Importance of Ions and Ionic Bonding in Living Things
As you learn about cells, you’ll see ions playing important roles in many biological processes.
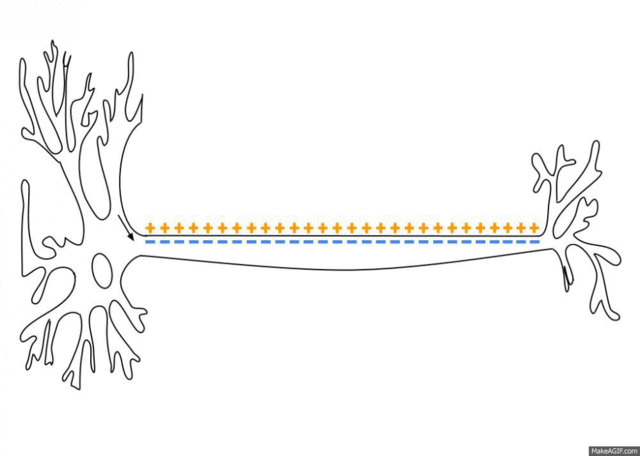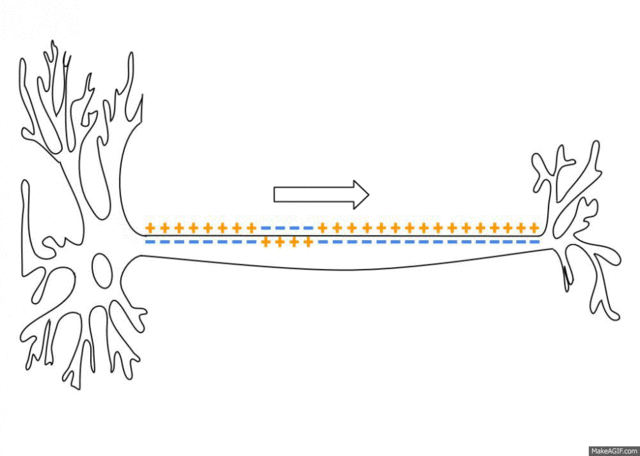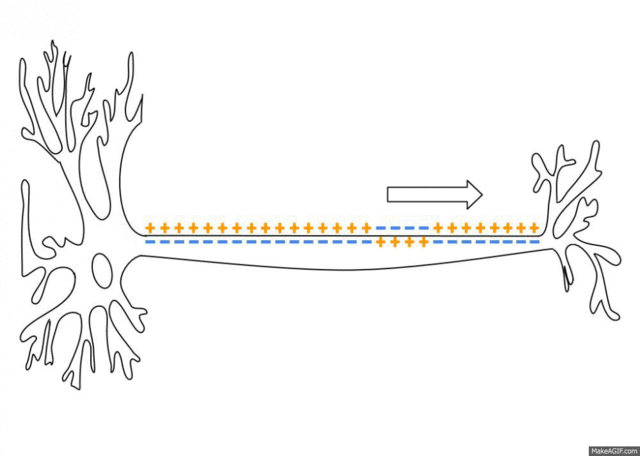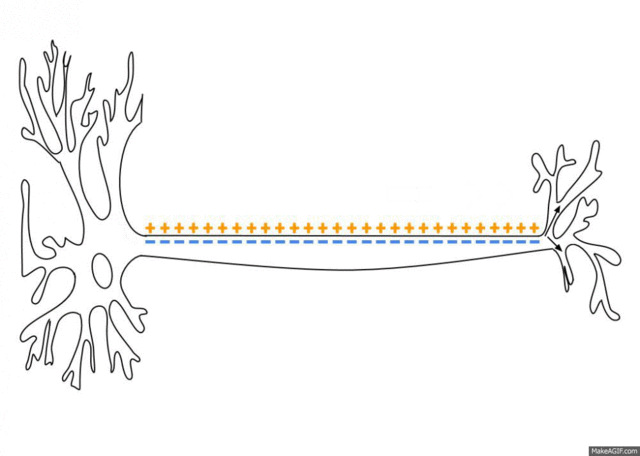
5.1: Ions and nerve cells
When you learn about how nerve cells transmit messages, for example, you’ll see that nerve impulses are waves of ions moving along the length of a nerve cell. That’s what’s happening in the image below.
5.2. Ions and the structure of molecules
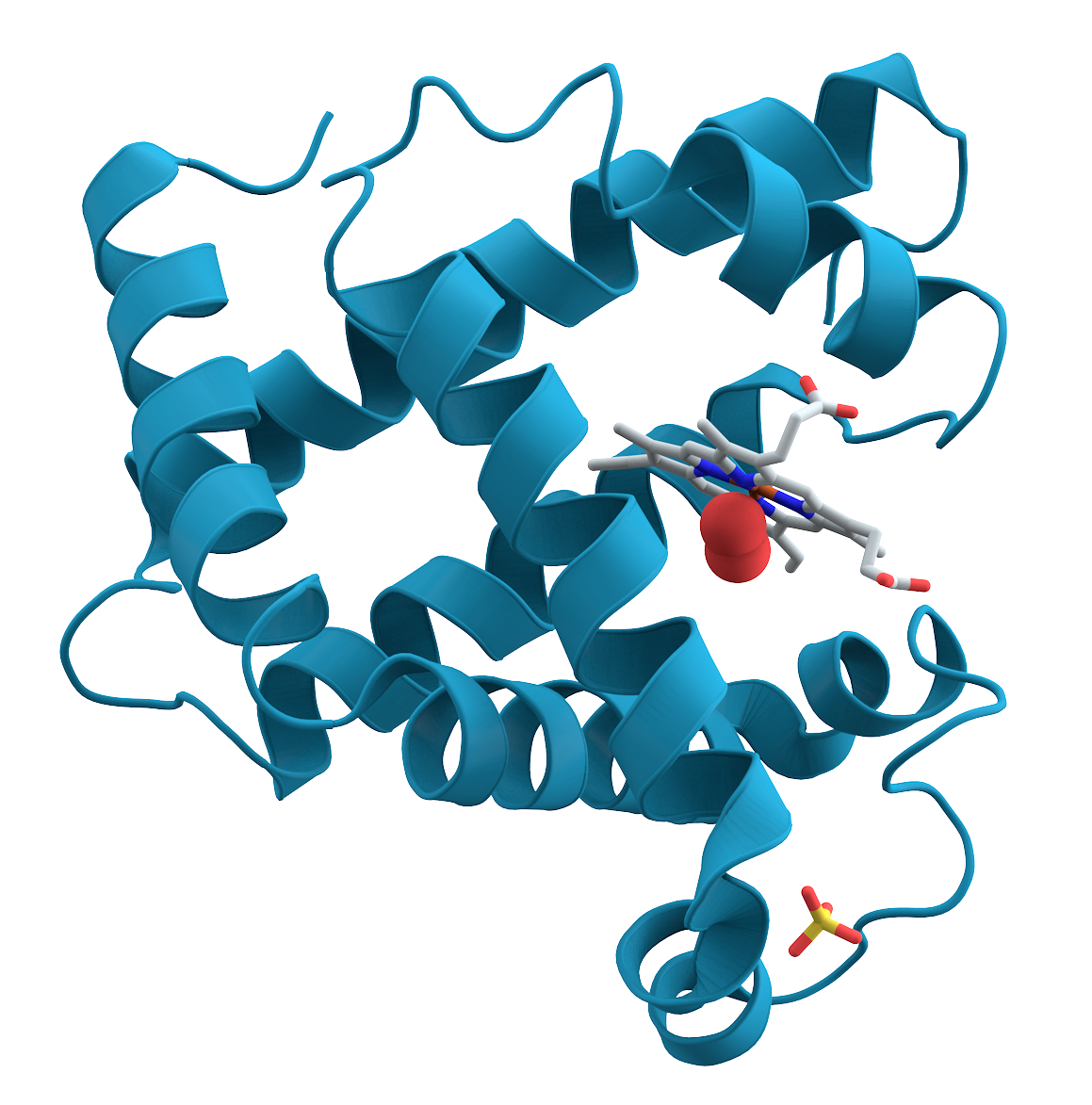
Ionic bonds play a key role in determining the structure of molecules.
- As we’ll see later in this course, a molecule’s shape defines its function.
- The job of the myoglobin molecule shown at the left is to store oxygen in muscle cells.
- Myoglobin can only do its job if it’s folded in the exact way shown.
- One of the reasons why it’s folded is because of an ionic bond within the molecule.
You’ll learn lots more about ions as we progress through this course.
6. Next steps
If you need more practice, please scroll up to the top and work through this tutorial again. Otherwise, proceed to Covalent Bonds (the next basic chemistry tutorial).
