Link to Chemistry for Biology Students: Study Guide/Student Worksheet
Introduction
Atoms (Basic Chemistry Tutorial 1) focused on the general structure of atoms. Here, you’ll learn how to determine an atom’s specific structure, which will set us up for learning about chemical bonds in the next tutorials.
If you’ve already watched the video, click here, or scroll down below the video to start interacting.
1. Drawing the Simplest Atoms: Hydrogen and Helium
The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass1. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system.
1 That’s not including “dark matter,” which is beyond the scope of this course.Hydrogen atoms have one proton and one electron. So we can represent hydrogen like this:
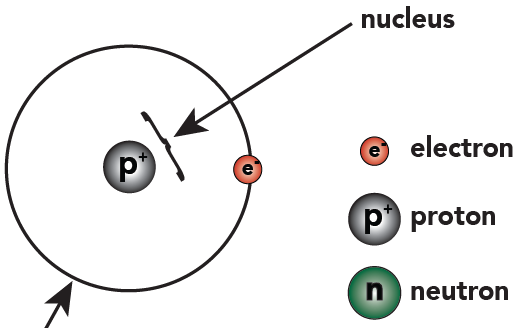
Hydrogen has one single proton that goes into the nucleus. Outside the nucleus orbits a single electron, which is found in the first energy level.
[qwiz style = “width: 528px; min-height:0px; border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Drawing Atoms, Predict Helium”]
[h]Predict!
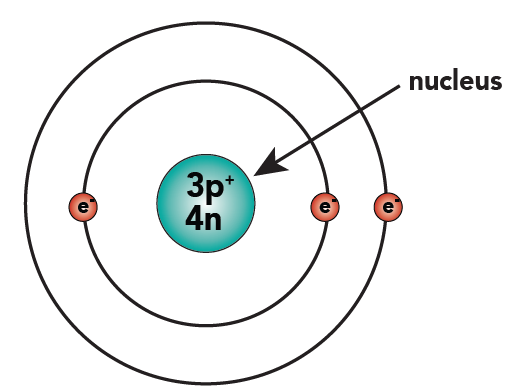
[q]Helium is the second most common element in the universe. Helium atoms have two protons, two neutrons, and two electrons. See if you can extend what we’ve learned so far to draw a diagram of helium. After you’ve drawn it, click “show the answer” to see if you drew it correctly.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==In helium, two protons and two neutrons are found in the nucleus. Two electrons orbit outside the nucleus, in the first energy level (also called a “shell” or “orbit”). Note that the term “orbital,” used in the accompanying video, has a slightly more technical meaning, and we’ll be avoiding it here.
[x]
[/qwiz]
Make sure you’ve clicked “show the answer” and studied the diagram above before proceeding.
2. The Octet Rule
For atoms with more than two protons and two electrons, a few rules need to be introduced to diagram them in a way that represents their chemical properties.1
1 When you learn about atoms in a chemistry class, you’ll learn a much more sophisticated and accurate model of electron arrangement. But for the biology that you’ll learn in an introductory high school (or even college) course, the rules that follow will work.The first rule is called the Octet Rule. “Octet” refers to eight electrons, as you’ll see below. The key parts of the rule are:
- Each of the energy levels where electrons are found has a fixed capacity to hold electrons.
- The first energy level can hold up to two electrons.
- The second and third energy levels can hold up to eight electrons (hence, “Octet”).
- When one energy level is filled, you start filling the next one.
Let’s use the Octet Rule to draw a lithium atom.
[qwiz style = “width: 528px; min-height:0px; border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Drawing Atoms, Predict Lithium”]
[h]Predict!
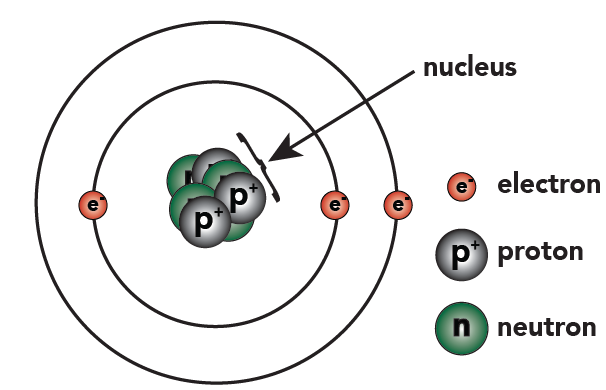
[q]Lithium is a metallic element that has 3 protons, 4 neutrons, and 3 electrons. The first two electrons go into the first energy level. With the first energy level filled, the next electron goes into the second energy level. Use the description of the Octet Rule to draw a diagram of lithium. After you’ve drawn it, click “show the answer” to see if you drew it correctly.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==In lithium, three protons and four neutrons are found in the nucleus. Two electrons orbit outside the nucleus, in the first energy level (also called a “shell”). The third electron goes into the second energy level.
[x]
[/qwiz]
Make sure you’ve clicked “show the answer” and studied the diagram above before proceeding.
From this point on we’re going to simplify our diagrams by drawing the nucleus differently.
- Instead of showing individual protons and neutrons, draw a circle to indicate the nucleus.
- Write the number of protons and neutrons inside the circle.
Below on the left, you can see this new representation of lithium, next to the one we’ve previously used.
| New representation of lithium (notice how protons and neutrons are indicated in the nucleus) | Previous representation of lithium that shows the individual protons and neutrons |
To represent the atoms of the next few elements, we need one more rule: place the electrons in the energy level singly until you have four, and then pair them up until you have filled the energy level with 8 electrons. As we’ll see, this rule will help you to understand what’s happening when we learn about chemical bonding, where electrons trade or share electrons. Following that rule, we can draw the next atoms, all the way through neon.
[qwiz style = “width: 528px; min-height:0px; border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Drawing Atoms, Predict B, C, and N”]
[h]Predict!
[q]Using what you know about the Octet Rule, draw three more atoms
- Boron: 5 protons, 6 neutrons, 5 electrons
- Carbon: 6 protons, 6 neutrons, 6 electrons
- Nitrogen: 7 protons, 7 neutrons, 7 electrons
After you’ve drawn them, click “show the answer” to see if you drew them correctly.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==| [Qq] | ||
| Two electrons go in the first energy level. The next three go into the second energy level. Each outer electron is unpaired. | Two electrons go in the first energy level. The next four go into the second energy level. Each outer electron is unpaired. | Two electrons go in the first energy level. The next five go into the second energy level. One of the outer electrons has to be paired up. |
[x]
[/qwiz]
Make sure you’ve studied the answer to the problem above before going on.
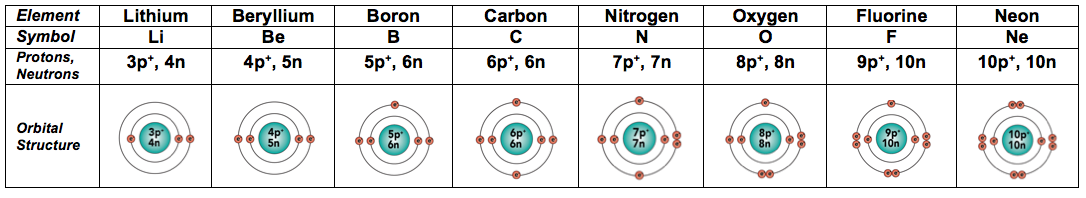
2a. Elements 3 through 10 (Lithium through Neon)
The table below shows all the atoms in the second row of the Period Table of the Elements. Study it and make sure that the arrangement of electrons makes sense to you before going on.
2b. Elements 11 through 20
If the number of electrons in an atom exceeds 10 (the limit of the first two energy levels), then the next electrons go into the third energy level. The rules for filling the third energy level are the same as those described above.
[qwiz style = “width: 528px; min-height:0px; border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Drawing Atoms, Predict Aluminum”]
[h]Predict!
[q]Aluminum has 13 protons, 14 neutrons, and 13 electrons. Use the description of the Octet Rule to draw a diagram of aluminum. After you’ve drawn it, click “show the answer” to see if you drew it correctly.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==To draw aluminum, put two electrons in the first energy level. The next eight go into the second energy level. The remaining 3 electrons go into the third energy level.
[x]
[/qwiz]
Here are elements 10 through 18, which are the ones in the third row of the Periodic Table. Make sure that their electron arrangement makes sense before reading on.
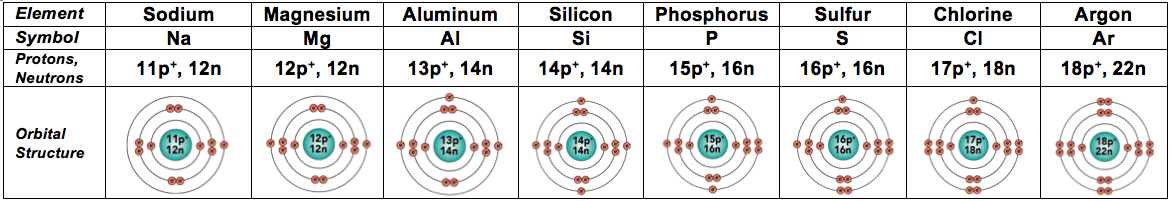
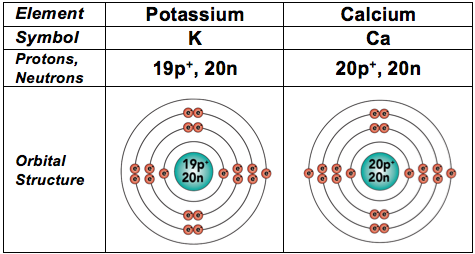
And because they’re also fairly common in living things, we’ll throw in the next two elements, too. In these elements, electrons start filling the fourth energy level.
3. Review: Four Things to Know About Drawing Atoms
In this tutorial, we’ve focused on understanding the structure of atoms. We’ve specifically learned that
- Electrons are found in energy levels outside the nucleus
- The first energy level has a capacity of two electrons
- The next two energy levels each have a capacity of 8 electrons.
- These rules about electron capacity are known as the Octet rule.
Using these rules, if you’re given the number of protons that an atom has, you should be able to figure out the arrangement of its electrons.
4. Checking Understanding (Flashcards: Atomic Structure)
To check your understanding, we’re going to start with a few flashcards, then move on to an interactive quiz.
[qdeck style=”border: 2px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Atomic Structure/Drawing Atoms Flashcards (M3)”]
[h] Flashcards: Atomic Structure
[i] Instructions
- Click ‘Check Answer’ to see the answer to each card.
- If you know it, click ‘Got it.”
- If you don’t know it as well as you’d like, click ‘Need more practice,’ and that card will go to the bottom of the deck so you can practice it again.
- ‘Shuffle’ lets you shuffle the deck.
[!]CARD 1 ++++++++++++++++++++++++++++++++++++++++++++[/!]
[q] Carbon has 6 protons, 6 neutrons, 6 electrons. On a sheet of paper, draw a carbon atom, then flip the card to see if you drew it correctly.
[a]
Carbon’s protons and neutrons go into the nucleus. The first two electrons go into the first energy level. The next four electrons go into the second energy level. To represent how carbon forms chemical bonds, it’s best to draw all four electrons as being unpaired.
[!]CARD 2 ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q] Sodium has 11 protons, 11 neutrons, 11 electrons. On a sheet of paper, draw a sodium atom, then flip the card to see if you drew it correctly.
[a]
Sodium’s protons and neutrons go into the nucleus. The first two electrons go into the first energy level. The next eight electrons go into the second energy level and are best shown as four pairs. The last electron goes into the third energy level.
[!]CARD 3 ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q] Oxygen has 8 protons, 8 neutrons, and 8 electrons. On a sheet of paper, draw an oxygen atom, then flip the card to see if you drew it correctly.
[a]
Oxygen’s protons and neutrons go into the nucleus. The first two electrons go into the first energy level. The next six electrons go into the second energy level. The best way to represent the electrons is to draw them as two pairs, and two more single electrons.
[!]CARD 4 ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q] Phosphorus has 15 protons, 15 neutrons, and 15 electrons. On a sheet of paper, draw a phosphorus atom, then flip the card to see if you drew it correctly.
Phosphorus’s protons and neutrons go into the nucleus. The first two electrons go into the first energy level. The next eight electrons go into the second energy level. The next five electrons go into the third.
[!]CARD 5 ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q] Potassium has 19 protons, 19 neutrons, 19 electrons. On a sheet of paper, draw a potassium atom, then flip the card to see if you drew it correctly.
Potassium’s protons and neutrons go into the nucleus. The first two electrons go into the first energy level. The next eight electrons go into the second energy level. The next eight electrons go into the third energy level. The last electron goes into the fourth energy level.
[!]CARD 6 ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++[/!]
[q] Nitrogen has 7 protons, 7 neutrons, and 7 electrons. On a sheet of paper, draw a nitrogen atom, then flip the card to see if you drew it correctly.
[a]
Nitrogen’s protons and neutrons go into the nucleus. The first two electrons go into the first energy level. The next five electrons go into the second energy level. The best way to represent the electrons is to draw them as one pair, and three single electrons.
[x]
If you want more practice with these terms or concepts, please press the restart button below. Otherwise, continue to the final quiz.
[restart]
[/qdeck]
5. Quiz: Structure of Atoms
This quiz tests you on all of the terms you’ve learned so far. It also tests you on your ability to correctly identify an atom’s structure.
[qwiz style = “border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Atomic Structure/Drawing Atoms Quiz (M3)”]
[h] QUIZ: Structure of Atoms
[i] This quiz covers key concepts and terms related to the structure of atoms.
Here’s how it works.
- The quiz consists of a series of multiple choice questions, but it behaves like a stack of flashcards.
- If you get a question right, you move on to the next question.
- If you get a question wrong, you’ll get an explanation, and then that question goes to the bottom of the stack.
- To exit the quiz, you have to answer each question correctly.
[q] The building block of matter is the
[c]IG51Y2xldXM=[Qq]
[c]IGF0 b20=[Qq]
[c]IHByb3Rvbg==[Qq]
[c]IGVsZWN0cm9uIA==[Qq]
[f]IE5vLiBUaGUgbnVjbGV1cyBpcyB0aGUgY2VudHJhbCBwYXJ0IG9mIHRoZSBidWlsZGluZyBibG9jayBvZiBtYXR0ZXIuIFdoYXQgd291bGQgeW91IGNhbGwgdGhlIHVuaXQgdGhhdCBtYWtlcyB1cCB0aGUgbnVjbGV1cyBwbHVzIHRoZSBlbGVjdHJvbnM/[Qq]
[f]IENvcnJlY3QuIEF0b21zIGFyZSB0aGUgYnVpbGRpbmcgYmxvY2tzIG9mIG1hdHRlci4=[Qq]
[f]IE5vLiBQcm90b25zIGFyZSBmb3VuZCBpbiB0aGUgbnVjbGV1cy4gV2hhdCB3b3VsZCB5b3UgY2FsbCB0aGUgdW5pdCB0aGF0IG1ha2VzIHVwIHRoZSBudWNsZXVzIHBsdXMgdGhlIGVsZWN0cm9ucz8=[Qq]
[f]IE5vLiBFbGVjdHJvbnMgb3JiaXQgb3V0c2lkZSB0aGUgbnVjbGV1cy4gV2hhdCB3b3VsZCB5b3UgY2FsbCB0aGUgdW5pdCB0aGF0IG1ha2VzIHVwIHRoZSBudWNsZXVzIHBsdXMgdGhlIGVsZWN0cm9ucz8=
Cg==[Qq]
[q] Almost all of an atom’s mass is in the
[c]IHByb3RvbnM=[Qq]
[c]IG5ldXRyb25z[Qq]
[c]IG51Y2 xldXM=[Qq]
[c]IGVsZWN0cm9uIGVuZXJneSBsZXZlbHM=[Qq]
[f]IE5vLiBQcm90b25zIGRvIG1ha2UgdXAgYSBzaWduaWZpY2FudCBwYXJ0IG9mIGFuIGF0b20mIzgyMTc7cyBtYXNzLCBidXQgYSBiZXR0ZXIgYW5zd2VyIHdvdWxkIGJlIHRoZSBwYXJ0IHRoYXQgaW5jbHVkZXMgYm90aCBwcm90b25zIGFuZCBuZXV0cm9ucy4gV2hhdCBwYXJ0IG9mIHRoZSBhdG9tIGlzIHRoYXQ/[Qq]
[f]IE5vLiBOZXV0cm9ucyBkbyBtYWtlIHVwIGEgc2lnbmlmaWNhbnQgcGFydCBvZiBhbiBhdG9tJiM4MjE3O3MgbWFzcywgYnV0IGEgYmV0dGVyIGFuc3dlciB3b3VsZCBiZSB0aGUgcGFydCB0aGF0IGluY2x1ZGVzIGJvdGggcHJvdG9ucyBhbmQgbmV1dHJvbnMuIFdoYXQgcGFydCBvZiB0aGUgYXRvbSBpcyB0aGF0Pw==[Qq]
[f]IFllcy4gQWxtb3N0IGFsbCBvZiBhbiBhdG9tJiM4MjE3O3MgbWFzcyBpcyBmb3VuZCBpbiB0aGUgbnVjbGV1cywgd2hpY2ggY29uc2lzdHMgb2YgcHJvdG9ucyBhbmQgbmV1dHJvbnMu[Qq]
[f]IE5vLiBBbiBlbmVyZ3kgbGV2ZWwgaXMgYSByZWdpb24gb3V0c2lkZSBvZiB0aGUgbnVjbGV1cyB3aGVyZSBlbGVjdHJvbnMgYXJlIGZvdW5kLiBXaGVyZSBkbyB5b3UgZmluZCB0aGUgcHJvdG9ucyBhbmQgbmV1dHJvbnMsIHRoZSBtb3N0IG1hc3NpdmUgcGFydGljbGVzIGluIGFuIGF0b20/
Cg==wqA=[Qq]
[q] The two particles found in the atom’s nucleus are the
[c]IG5ldXRyb24gYW 5kIHByb3Rvbg==[Qq]
[c]IG5ldXRyb24gYW5kIGVsZWN0cm9u[Qq]
[c]IHByb3RvbiBhbmQgZWxlY3Ryb24=[Qq]
[f]IENvcnJlY3QuIE5ldXRyb25zIGFuZCBwcm90b25zIG1ha2UgdXAgdGhlIGF0b20mIzgyMTc7cyBudWNsZXVzLg==[Qq]
[f]IE5vLiBOZXV0cm9ucyBhcmUgZm91bmQgaW4gdGhlIG51Y2xldXMsIGJ1dCBub3QgZWxlY3Ryb25zLiBUaGUgc2Vjb25kIHBhcnRpY2xlIGlzIG9uZSB3aXRoIGEgcG9zaXRpdmUgY2hhcmdlLg==[Qq]
[f]IE5vLiBQcm90b25zIGFyZSBmb3VuZCBpbiB0aGUgbnVjbGV1cywgYnV0IG5vdCBlbGVjdHJvbnMuIFRoZSBzZWNvbmQgcGFydGljbGUgaXMgdGhlIG9uZSB3aXRoIGEgbmV1dHJhbCBjaGFyZ2UuwqA=
Cg==wqA=[Qq]
[q] The positively charged particle in an atom is the
[c]IGVsZWN0cm9u[Qq]
[c]IHByb3 Rvbg==[Qq]
[c]IG5ldXRyb24=[Qq]
[f]IE5vLiBFbGVjdHJvbnMgaGF2ZSBhIG5lZ2F0aXZlIGNoYXJnZS4gVGhlIHBvc2l0aXZlbHkgY2hhcmdlZCBwYXJ0aWNsZSBpcyBmb3VuZCBpbiB0aGUgbnVjbGV1cy4=[Qq]
[f]IENvcnJlY3QuIFRoZSBwb3NpdGl2ZWx5IGNoYXJnZWQgcGFydGljbGUgaW4gdGhlIGF0b20gaXMgdGhlIHByb3Rvbi4=[Qq]
[f]IE5vLiBOZXV0cm9ucyBoYXZlIG5vIGNoYXJnZSAobWFraW5nIHRoZW0gbmV1dHJhbCkuIFRoZSBwb3NpdGl2ZWx5IGNoYXJnZWQgcGFydGljbGUgaXMgdGhlIG90aGVyIHBhcnRpY2xlIChiZXNpZGVzIHRoZSBuZXV0cm9uKSBpbiB0aGUgbnVjbGV1cy4=
Cg==wqA=[Qq]
[q] The negatively charged particle in an atom is the
[c]IGVsZW N0cm9u[Qq]
[c]IHByb3Rvbg==[Qq]
[c]IG5ldXRyb24=[Qq]
[f]IFllcy4gRWxlY3Ryb25zIGhhdmUgYSBuZWdhdGl2ZSBjaGFyZ2UuIA==[Qq]
[f]IE5vLiBQcm90b25zIGhhdmUgYSBwb3NpdGl2ZSBjaGFyZ2UuIFRoZSBuZWdhdGl2ZWx5IGNoYXJnZWQgcGFydGljbGUgaW4gdGhlIGF0b20gaXMgdGhlIG9uZSBvdXRzaWRlIHRoZSBudWNsZXVzLg==[Qq]
[f]IE5vLiBOZXV0cm9ucyBoYXZlIG5vIGNoYXJnZSAobWFraW5nIHRoZW0gbmV1dHJhbCkuIFRoZSBuZWdhdGl2ZWx5IGNoYXJnZWQgcGFydGljbGUgaW4gdGhlIGF0b20gaXMgdGhlIG9uZSBvdXRzaWRlIHRoZSBudWNsZXVzLg==
Cg==wqA=[Qq]
[q] The particle in an atom that has no charge is the
[c]IGVsZWN0cm9u[Qq]
[c]IHByb3Rvbg==[Qq]
[c]IG5ldX Ryb24=[Qq]
[f]wqAgTm8uIEVsZWN0cm9ucyBoYXZlIGEgbmVnYXRpdmUgY2hhcmdlLiA=[Qq]
[f]IE5vLiBQcm90b25zIGhhdmUgYSBwb3NpdGl2ZSBjaGFyZ2UuIFRoZSBwYXJ0aWNsZSB3aXRob3V0IGFueSBjaGFyZ2UgaXMgdGhlIG90aGVyIHBhcnRpY2xlIChiZXNpZGVzIHRoZSBwcm90b24pIGluIHRoZSBudWNsZXVzLiA=[Qq]
[f]IEV4YWN0bHkuIE5ldXRyb25zIGhhdmUgbm8gY2hhcmdlIChtYWtpbmcgdGhlbSBuZXV0cmFsKS4=
Cg==[Qq]
[q] Electrons are found in
[c]IHRoZSBudWNsZXVz[Qq]
[c]IGVuZXJneSBsZXZl bHMgb3Igc2hlbGxz[Qq]
[f]IE5vLiBUaGUgbnVjbGV1cyBpcyB0aGUgY2VudHJhbCBwYXJ0IG9mIHRoZSBhdG9tLCB3aGVyZSBwcm90b25zIGFuZCBuZXV0cm9ucyBhcmUgZm91bmQu[Qq]
[f]IENvcnJlY3QuIEVsZWN0cm9ucyBhcmUgZm91bmQgaW4gZW5lcmd5IGxldmVscyAoYWxzbyBjYWxsZWTCoA==c2hlbGxzKQ==
Cg==[Qq]
[q] Of the terms below, the one most similar to “orbit” is
[c]IGVuZXJneS BsZXZlbA==[Qq]
[c]IG51Y2xldXM=[Qq]
[f]IFllcy4gVGhlIHRlcm1zICYjODIxNjtlbmVyZ3kgbGV2ZWwmIzgyMTc7IGFuZCAmIzgyMTY7b3JiaXQmIzgyMTc7IGJvdGggcmVmZXIgdG8gdGhlIHJlZ2lvbiBvdXRzaWRlIG9mIHRoZSBudWNsZXVzIHdoZXJlIGVsZWN0cm9ucyBhcmUgZm91bmQuIA==[Qq]
[f]IE5vLiBUaGUgbnVjbGV1cyBpcyB0aGUgY2VudHJhbCByZWdpb24gb2YgdGhlIGF0b20sIHdoZXJlIHByb3RvbnMgYW5kIG5ldXRyb25zIGFyZSBmb3VuZC4gWW91JiM4MjE3O3JlIGxvb2tpbmcgZm9yIGEgdGVybSB0aGF0IHJlZmVycyB0byB3aGVyZSBlbGVjdHJvbnMgYXJlIGZvdW5kLg==
Cg==wqA=[Qq]
[q] In an uncharged atom, the number of protons is equal to the number of
[c]IGVuZXJneSBsZXZlbHM=[Qq]
[c]IHNoZWxscw==[Qq]
[c]IG5ldXRyb25z[Qq]
[c]IGVsZWN0 cm9ucw==[Qq]
[f]IE5vLiBXaGlsZSB0aGUgbnVtYmVyIG9mIHByb3RvbnMgY2FuIGVxdWFsIHRoZSBudW1iZXIgb2YgZW5lcmd5IGxldmVscywgaXQmIzgyMTc7cyBub3QgYSBuZWNlc3NhcnkgcmVsYXRpb25zaGlwLiBUaGluayBhYm91dCBhIHBhcnRpY2xlIHdob3NlIGNoYXJnZSBpcyB0aGUgb3Bwb3NpdGUgb2YgdGhlIHByb3RvbiYjODIxNztzIGNoYXJnZS4=[Qq]
[f]IE5vLiBXaGlsZSB0aGUgbnVtYmVyIG9mIHByb3RvbnMgY2FuIGVxdWFsIHRoZSBudW1iZXIgb2YgZWxlY3Ryb24gc2hlbGxzLCBpdCYjODIxNztzIG5vdCBhIG5lY2Vzc2FyeSByZWxhdGlvbnNoaXAuIFRoaW5rIGFib3V0IGEgcGFydGljbGUgd2hvc2UgY2hhcmdlIGlzIHRoZSBvcHBvc2l0ZSBvZiB0aGUgcHJvdG9uJiM4MjE3O3MgY2hhcmdlLg==[Qq]
[f]IE5vLiBXaGlsZSBpdCYjODIxNztzIG5vdCB1bmNvbW1vbiBmb3IgdGhlIG51bWJlciBvZiBwcm90b25zIHRvIGVxdWFsIHRoZSBudW1iZXIgb2YgbmV1dHJvbnMsIHRoZSBudW1iZXIgY2FuIGFsc28gYmUgZGlmZmVyZW50LiBUaGluayBhYm91dCBhIHBhcnRpY2xlIHdob3NlIGNoYXJnZSBpcyB0aGUgb3Bwb3NpdGUgb2YgdGhlIHByb3RvbiYjODIxNztzIGNoYXJnZS8=[Qq]
[f]IEV4Y2VsbGVudCEgSW4gYW4gdW5jaGFyZ2VkIGF0b20sIHRoZSBudW1iZXIgb2YgcHJvdG9ucyBpcyBlcXVhbCB0byB0aGUgbnVtYmVyIG9mIGVsZWN0cm9ucy4g
Cg==wqA=[Qq]
[q] An element is composed of
[c]IE9ubHkgb25lIHR5 cGUgb2YgYXRvbQ==[Qq]
[c]IEF0IGxlYXN0IHR3byB0eXBlcyBvZiBhdG9tcw==[Qq]
[c]IE9ubHkgYXRvbXMgdGhhdCBoYXZlIGxvc3QgdGhlaXIgZWxlY3Ryb25z[Qq]
[f]IFllcy4gQW4gZWxlbWVudCBpcyBhIHN1YnN0YW5jZSB0aGF0IGlzIGNvbXBvc2VkIG9mIG9ubHkgb25lIHR5cGUgb2YgYXRvbS4=[Qq]
[f]IE5vLiBTdWJzdGFuY2VzIGNvbXBvc2VkIG9mIHR3byBvciBtb3JlIHR5cGVzIG9mIGF0b21zIGFyZSBjb21wb3VuZHMgKGFuZCB3ZSYjODIxNztsbCBsZWFybiBtb3JlIGFib3V0IHRoZW0gbGF0ZXIu[Qq]
[f]IE5vLiBXaGlsZSBhbiBhdG9tIG1pZ2h0IHRlbXBvcmFyaWx5IGxvc2UgaXRzIGVsZWN0cm9ucyAobW9yZSBhYm91dCB0aGF0IGxhdGVyKSwgdGhlcmUgYXJlIG5vIHN1YnN0YW5jZXMgdGhhdCBhcmUgY29tcG9zZWQgb2YgYXRvbXMgdGhhdCBoYXZlIGxvc3QgdGhlaXIgZWxlY3Ryb25zLg==
Cg==wqA=[Qq]
[q] Which of the following could NOT be the chemical symbol for an element
[c]IEZl[Qq]
[c]IEhl Zw==[Qq]
[c]IEk=[Qq]
[c]IE1u[Qq]
[f]IE5vLiBDaGVtaWNhbCBzeW1ib2xzIGNvbnNpc3Qgb2Ygb25lIG9yIHR3byBsZXR0ZXJzLiBUaGUgZmlyc3QgKG9yIG9ubHkpIGxldHRlciBpcyBjYXBpdGFsaXplZC4gVGhlIHNlY29uZCBsZXR0ZXIgaXMgbG93ZXJjYXNlLiAmIzgyMjA7RmUmIzgyMjE7IGlzIGEgbGVnaXRpbWF0ZSBzeW1ib2wmIzgyMzA7dGhlIHN5bWJvbCBmb3IgdGhlIGxldHRlciBJcm9uLiA=[Qq]
[f]IFllczogJiM4MjIwO0hlZyYjODIyMTsgY291bGQgTk9UIGJlIGEgY2hlbWljYWwgc3ltYm9sIGZvciBhbiBlbGVtZW50LiBDaGVtaWNhbCBzeW1ib2xzIGNvbnNpc3Qgb2Ygb25lIG9yIHR3byBsZXR0ZXJzLiBUaGUgZmlyc3QgKG9yIG9ubHkpIGxldHRlciBpcyBjYXBpdGFsaXplZC4gVGhlIHNlY29uZCBsZXR0ZXIgaXMgbG93ZXJjYXNlLiBXaXRoIHRocmVlIGxldHRlcnMsICYjODIyMDtIZWcmIzgyMjE7IGlzIG5vdCBhIGxlZ2l0aW1hdGUgY2hlbWljYWwgc3ltYm9sLsKg[Qq]
[f]IE5vLiBDaGVtaWNhbCBzeW1ib2xzIGNvbnNpc3Qgb2Ygb25lIG9yIHR3byBsZXR0ZXJzLiBUaGUgZmlyc3QgKG9yIG9ubHkpIGxldHRlciBpcyBjYXBpdGFsaXplZC4gVGhlIHNlY29uZCBsZXR0ZXIgaXMgbG93ZXJjYXNlLiAmIzgyMjA7SSYjODIyMTsgaXMgYSBsZWdpdGltYXRlIHN5bWJvbCYjODIzMDt0aGUgc3ltYm9sIGZvciB0aGUgZWxlbWVudCBJb2RpbmUu[Qq]
[f]IE5vLiBDaGVtaWNhbCBzeW1ib2xzIGNvbnNpc3Qgb2Ygb25lIG9yIHR3byBsZXR0ZXJzLiBUaGUgZmlyc3QgKG9yIG9ubHkpIGxldHRlciBpcyBjYXBpdGFsaXplZC4gVGhlIHNlY29uZCBsZXR0ZXIgaXMgbG93ZXJjYXNlLiAmIzgyMjA7TW4mIzgyMjE7IGlzIGEgbGVnaXRpbWF0ZSBzeW1ib2wmIzgyMzA7dGhlIHN5bWJvbCBmb3IgdGhlIGVsZW1lbnQgTWFuZ2FuZXNl
Cg==wqA=[Qq]
[q] Nitrogen has 7 protons, 7 neutrons, and 7 electrons. Following the Octet Rule for placing electrons in energy levels, which of the following arrangements is correct?
[c]wqBBbGwgc2V2ZW4gZWxlY3Ryb25zIGluIHRoZSBmaXJzdMKgZW5lcmd5IGxldmVsLsKg[Qq]
[c]IFRocmVlIGVsZWN0cm9ucyBpbiB0aGUgZmlyc3QgZW5lcmd5IGxldmVsIGFuZCBmb3VyIGVsZWN0cm9ucyBpbiB0aGUgc2Vjb25kIGVuZXJneSBsZXZlbC7CoA==[Qq]
[c]wqBUd28gZWxlY3Ryb25zIGluIHRoZSBmaXJzdMKgZW5lcmd5IGxldm VsLCBmaXZlIGluIHRoZSBzZWNvbmTCoGVuZXJneSBsZXZlbC7CoA==[Qq]
[f]IE5vLiBUbyByZXByZXNlbnQgdGhlIHN0cnVjdHVyZSBvZiB0aGUgZWxlY3Ryb25zIGluIGFuIGF0b20sIGtlZXAgaW4gbWluZCB0aGUgY2FwYWNpdHkgb2YgZWFjaCBlbmVyZ3kgbGV2ZWwgKHR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0LCBlaWdodCBpbiB0aGUgc2Vjb25kLCBhbmQgZWlnaHQgaW4gdGhlIHRoaXJkLiBJbiBOaXRyb2dlbiwgd2l0aCBzZXZlbiBlbGVjdHJvbnMsIHRoZSBmaXJzdCB0d28gZWxlY3Ryb25zIG5lZWQgdG8gZ28gaW50byB0aGUgZmlyc3QgZW5lcmd5IGxldmVsLiBUaGF0IGxlYXZlcyBmaXZlIGVsZWN0cm9ucyBmb3IgdGhlIHNlY29uZCBlbmVyZ3kgbGV2ZWwu[Qq]
[f]IE5vLiBUbyByZXByZXNlbnQgdGhlIHN0cnVjdHVyZSBvZiB0aGUgZWxlY3Ryb25zIGluIGFuIGF0b20sIGtlZXAgaW4gbWluZCB0aGUgY2FwYWNpdHkgb2YgZWFjaCBlbmVyZ3kgbGV2ZWwgKHR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0LCBlaWdodCBpbiB0aGUgc2Vjb25kLCBhbmQgZWlnaHQgaW4gdGhlIHRoaXJkLiBJbiBOaXRyb2dlbiwgd2l0aCBzZXZlbiBlbGVjdHJvbnMsIHRoZSBmaXJzdCB0d28gZWxlY3Ryb25zIG5lZWQgdG8gZ28gaW50byB0aGUgZmlyc3QgZW5lcmd5IGxldmVsLiBUaGF0IGxlYXZlcyBmaXZlIGVsZWN0cm9ucyBmb3IgdGhlIHNlY29uZCBlbmVyZ3kgbGV2ZWwu[Qq]
[f]IENvcnJlY3QuIEluIE5pdHJvZ2VuLCB3aXRoIHNldmVuIGVsZWN0cm9ucywgdGhlIGZpcnN0IHR3byBlbGVjdHJvbnMgbmVlZCB0byBnbyBpbnRvIHRoZSBmaXJzdMKgZW5lcmd5IGxldmVsLiBUaGF0IGxlYXZlcyBmaXZlIGVsZWN0cm9ucyBmb3IgdGhlIHNlY29uZMKgZW5lcmd5IGxldmVsLg==
Cg==
[q] Sulfur has 16 protons, 16 neutrons, and 16 electrons. Following the Octet Rule for placing electrons in energy level, which of the following arrangements is correct?
[c]IFR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0IGVuZXJneSBsZXZlbCwgZWlnaHQgZWxlY3Ryb25zIGluIH RoZSBzZWNvbmTCoGVuZXJneSBsZXZlbCwgYW5kIHNpeCBpbiB0aGUgdGhpcmTCoGVuZXJneSBsZXZlbC4=[Qq]
[c]wqBFaWdodCBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0wqBlbmVyZ3kgbGV2ZWwsIGVpZ2h0IGVsZWN0cm9ucyBpbiB0aGUgc2Vjb25kwqBlbmVyZ3kgbGV2ZWwuwqA=[Qq]
[c]wqBTaXggZWxlY3Ryb25zIGluIHRoZSBmaXJzdMKgZW5lcmd5IGxldmVsLCBzaXggaW4gdGhlIHNlY29uZMKgZW5lcmd5IGxldmVsLCBhbmQgZm91ciBpbiB0aGUgdGhpcmQuwqA=[Qq]
[f]IEV4Y2VsbGVudC4gSW4gU3VsZnVyLCB3aXRoIDE2IGVsZWN0cm9ucywgdGhlIGZpcnN0IHR3byBlbGVjdHJvbnMgZ28gaW4gdGhlIGZpcnN0wqBlbmVyZ3kgbGV2ZWwsIHRoZSBuZXh0IDggaW4gdGhlIHNlY29uZMKgZW5lcmd5IGxldmVsLCBhbmQgdGhlIGxhc3Qgc2l4IGluIHRoZSB0aGlyZMKgZW5lcmd5IGxldmVsLg==
Cg==[Qq]
[f]IE5vLiBUbyByZXByZXNlbnQgdGhlIHN0cnVjdHVyZSBvZiBlbGVjdHJvbnMgaW4gYXRvbXMsIGtlZXAgaW4gbWluZCB0aGUgY2FwYWNpdHkgb2YgZWFjaCBlbmVyZ3kgbGV2ZWwgKHR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0LCBlaWdodCBpbiB0aGUgc2Vjb25kLCBhbmQgZWlnaHQgaW4gdGhlIHRoaXJkKS4gSW4gc3VsZnVyLCB3aXRoIDE2IGVsZWN0cm9ucywgdGhlIGZpcnN0IHR3byBlbGVjdHJvbnMgbmVlZCB0byBnbyBpbnRvIHRoZSBmaXJzdCBlbmVyZ3kgbGV2ZWwuIFRoZSBuZXh0IGVpZ2h0IHdvdWxkIGdvIGludG8gdGhlIHNlY29uZCBlbmVyZ3kgbGV2ZWwsIHdoaWNoIGxlYXZlcyBzaXggZWxlY3Ryb25zIGZvciB0aGUgdGhpcmQgZW5lcmd5IGxldmVsLg==[Qq]
[f]IE5vLiBUbyByZXByZXNlbnQgdGhlIHN0cnVjdHVyZSBvZiBlbGVjdHJvbnMgaW4gYXRvbXMsIGtlZXAgaW4gbWluZCB0aGUgY2FwYWNpdHkgb2YgZWFjaCBlbmVyZ3kgbGV2ZWwgKHR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0LCBlaWdodCBpbiB0aGUgc2Vjb25kLCBhbmQgZWlnaHQgaW4gdGhlIHRoaXJkKS4gSW4gc3VsZnVyLCB3aXRoIDE2IGVsZWN0cm9ucywgdGhlIGZpcnN0IHR3byBlbGVjdHJvbnMgbmVlZCB0byBnbyBpbnRvIHRoZSBmaXJzdCBlbmVyZ3kgbGV2ZWwuIFRoZSBuZXh0IGVpZ2h0IHdvdWxkIGdvIGludG8gdGhlIHNlY29uZCBlbmVyZ3kgbGV2ZWwsIHdoaWNoIGxlYXZlcyBzaXggZWxlY3Ryb25zIGZvciB0aGUgdGhpcmQgZW5lcmd5IGxldmVsLg==
Cg==wqA=[Qq]
[q] Carbon has 6 protons, 6 neutrons, and 6 electrons. Following the Octet Rule for placing electrons in energy level, which of the following arrangements is correct?
[c]wqBUaHJlZSBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0wqBlbmVyZ3kgbGV2ZWwsIGFuZCB0aHJlZSBpbiB0aGUgc2Vjb25kLg==[Qq]
[c]wqBPbmUgZWxlY3Ryb24gaW4gdGhlIGZpcnN0wqBlbmVyZ3kgbGV2ZWwsIGFuZCBmaXZlIGluIHRoZSBzZWNvbmQuwqA=[Qq]
[c]wqBUd28gZWxlY3Ryb25zIGluIHRoZSBmaXJzdMKgZW5lcm d5IGxldmVsLCBhbmQgZm91ciBpbiB0aGUgc2Vjb25kLsKg[Qq]
[f]IE5vLiBUbyByZXByZXNlbnQgdGhlIHN0cnVjdHVyZSBvZiBlbGVjdHJvbnMgaW4gYXRvbXMsIGtlZXAgaW4gbWluZCB0aGUgY2FwYWNpdHkgb2YgZWFjaCBlbmVyZ3kgbGV2ZWwgKHR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0LCBlaWdodCBpbiB0aGUgc2Vjb25kLCBhbmQgZWlnaHQgaW4gdGhlIHRoaXJkKS4gSW4gQ2FyYm9uLCB3aXRoIDYgZWxlY3Ryb25zLCB0aGUgZmlyc3QgdHdvIGVsZWN0cm9ucyBuZWVkIHRvIGdvIGludG8gdGhlIGZpcnN0IGVuZXJneSBsZXZlbC4gVGhlIG5leHQgZm91ciBnbyBpbnRvIHRoZSBzZWNvbmQgZW5lcmd5IGxldmVsLg==[Qq]
[f]IE5vLiBUbyByZXByZXNlbnQgdGhlIHN0cnVjdHVyZSBvZiBlbGVjdHJvbnMgaW4gYXRvbXMsIGtlZXAgaW4gbWluZCB0aGUgY2FwYWNpdHkgb2YgZWFjaCBlbmVyZ3kgbGV2ZWwgKHR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0LCBlaWdodCBpbiB0aGUgc2Vjb25kLCBhbmQgZWlnaHQgaW4gdGhlIHRoaXJkKS4gSW4gQ2FyYm9uLCB3aXRoIDYgZWxlY3Ryb25zLCB0aGUgZmlyc3QgdHdvIGVsZWN0cm9ucyBuZWVkIHRvIGdvIGludG8gdGhlIGZpcnN0IGVuZXJneSBsZXZlbC4gVGhlIG5leHQgZm91ciBnbyBpbnRvIHRoZSBzZWNvbmQgZW5lcmd5IGxldmVsLg==[Qq]
[f]IEV4Y2VsbGVudC4gSW4gQ2FyYm9uLCB3aXRoIDYgZWxlY3Ryb25zLCB0aGUgZmlyc3QgdHdvIGVsZWN0cm9ucyBnbyBpbnRvIHRoZSBmaXJzdCBlbmVyZ3kgbGV2ZWwsIGFuZCB0aGUgbmV4dCA0IGdvIGludG8gdGhlIHNlY29uZCBlbmVyZ3kgbGV2ZWwu
Cg==
[q] Potassium has 19 protons, 19 neutrons, and 19 electrons. Following the Octet Rule for placing electrons in energy level, which of the following arrangements is correct?
[c]wqBUZW4gZWxlY3Ryb25zIGluIHRoZSBmaXJzdMKgZW5lcmd5IGxldmVsLCBuaW5lIGluIHRoZSBzZWNvbmQu[Qq]
[c]IFR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0wqBlbmVyZ3kgbGV2ZWwsIGVpZ2h0IGluIHRo ZSBzZWNvbmQsIGVpZ2h0IGluIHRoZSB0aGlyZCwgYW5kIG9uZSBpbiB0aGUgZm91cnRoLg==[Qq]
[c]wqBUd28gZWxlY3Ryb25zIGluIHRoZSBmaXJzdMKgZW5lcmd5IGxldmVsLCB0ZW7CoGluIHRoZSBzZWNvbmQsIGFuZCBzZXZlbiBpbiB0aGUgdGhpcmQu[Qq]
[f]IE5vLiBUbyByZXByZXNlbnQgdGhlIHN0cnVjdHVyZSBvZiBlbGVjdHJvbnMgaW4gYXRvbXMsIGtlZXAgaW4gbWluZCB0aGUgY2FwYWNpdHkgb2YgZWFjaCBlbmVyZ3kgbGV2ZWwgKHR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0LCBlaWdodCBpbiB0aGUgc2Vjb25kLCBhbmQgZWlnaHQgaW4gdGhlIHRoaXJkKS4gSW4gUG90YXNzaXVtLCB3aXRoIDE5IGVsZWN0cm9ucywgdGhlIGZpcnN0IHR3byBlbGVjdHJvbnMgZ28gaW50byB0aGUgZmlyc3QgZW5lcmd5IGxldmVsLiBUaGUgbmV4dCA4IGdvIGludG8gdGhlIHNlY29uZCBlbmVyZ3kgbGV2ZWwuIFRoZSBuZXh0IDggZ28gaW50byB0aGUgdGhpcmQgZW5lcmd5IGxldmVsLiBUaGF0IGxlYXZlcyBvbmUgZWxlY3Ryb24gZm9yIHRoZSBmb3VydGggZW5lcmd5IGxldmVsLiA=[Qq]
[f]IE5pY2Ugam9iISBJbiBQb3Rhc3NpdW0sIHdpdGggMTkgZWxlY3Ryb25zLCB0aGUgZmlyc3QgdHdvIGVsZWN0cm9ucyBnbyBpbnRvIHRoZSBmaXJzdMKgZW5lcmd5IGxldmVsLiBUaGUgbmV4dCA4IGdvIGludG8gdGhlIHNlY29uZMKgZW5lcmd5IGxldmVsLiBUaGUgbmV4dCA4IGdvIGludG8gdGhlIHRoaXJkwqBlbmVyZ3kgbGV2ZWwuIFRoYXQgbGVhdmVzIG9uZSBlbGVjdHJvbiBmb3IgdGhlIGZvdXJ0aMKgZW5lcmd5IGxldmVsLg==
Cg==[f]IE5vLiBUbyByZXByZXNlbnQgdGhlIHN0cnVjdHVyZSBvZiBlbGVjdHJvbnMgaW4gYXRvbXMsIGtlZXAgaW4gbWluZCB0aGUgY2FwYWNpdHkgb2YgZWFjaCBlbmVyZ3kgbGV2ZWwgKHR3byBlbGVjdHJvbnMgaW4gdGhlIGZpcnN0LCBlaWdodCBpbiB0aGUgc2Vjb25kLCBhbmQgZWlnaHQgaW4gdGhlIHRoaXJkKS4gSW4gUG90YXNzaXVtLCB3aXRoIDE5IGVsZWN0cm9ucywgdGhlIGZpcnN0IHR3byBlbGVjdHJvbnMgZ28gaW50byB0aGUgZmlyc3QgZW5lcmd5IGxldmVsLiBUaGUgbmV4dCA4IGdvIGludG8gdGhlIHNlY29uZCBlbmVyZ3kgbGV2ZWwuIFRoZSBuZXh0IDggZ28gaW50byB0aGUgdGhpcmQgZW5lcmd5IGxldmVsLiBUaGF0IGxlYXZlcyBvbmUgZWxlY3Ryb24gZm9yIHRoZSBmb3VydGggZW5lcmd5IGxldmVsLsKg[Qq]
[x]
If you want to take this quiz again, click the button below
[restart]
[/qwiz]
6. Next steps
If you need more practice, please scroll up to the top and work through this tutorial again. Otherwise, continue to the next tutorial: Elements, Compounds, and Molecules.