Link to Chemistry for Biology Students: Study Guide/Student Worksheet
If you’ve already watched the video, click here, or scroll down below the video to start interacting.
1. Interactive Reading: Understanding Atoms
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Understanding Atoms, Interactive Reading (M3)”]
[h]Interactive Reading: Understanding Atoms
[i]The reading that follows includes fill-in-the-blanks, multiple-choice, and true-false questions. Reading like this will deepen your understanding of atoms. Enjoy!
[q labels = “top”]
1. Atoms are the building blocks of matter
Living things (just like everything else) are made up of atoms. Atoms are the _______________________. In terms of size, they’re incredibly small: the radius of a nitrogen atom is less than 1/10th of 1 billionth of a meter. If this analogy is helpful, think of how much smaller a blueberry is than the entire Earth is. That’s how much _________ an atom is than a blueberry.
[l]smaller
[fx] No. Please try again.
[f*] Good!
[l]building blocks of matter
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[q]
2. Atoms are made of protons, neutrons, and electrons
Almost all of the mass of an atom is contained in its nucleus. The nucleus consists of protons, which have a positive electrical charge, and neutrons, which have no charge. Far away from the atom’s nucleus are negatively charged particles called electrons.
In the model of a carbon atom shown here, the atom is shown as a type of miniature solar system. The nucleus is where the sun would be (in the center), and the electrons are shown as planets orbiting outside. Note that the reality is much trickier, but what’s here works just fine for understanding high school, AP, or even introductory college-level biology.
Answer this question to continue:
The charge on a proton is
[c]cG9zaX RpdmU=[Qq]
[c]bmVnYXRpdmU=[Qq]
[c]bmV1dHJhbA==[Qq]
[f]Q29ycmVjdC4gUHJvdG9ucyBoYXZlIGEgcG9zaXRpdmUgY2hhcmdlLg==[Qq]
[f]Tm8uIFByb3RvbnMgaGF2ZSBhIHBvc2l0aXZlIGNoYXJnZS4gVGhlIHBhcnRpY2xlcyB0aGF0IGhhdmUgYSBuZWdhdGl2ZSBjaGFyZ2UgYXJlIGVsZWN0cm9ucy4=[Qq]
[f]Tm8uIFByb3RvbnMgaGF2ZSBhIHBvc2l0aXZlIGNoYXJnZS4gVGhlIHBhcnRpY2xlcyB0aGF0IGhhdmUgYSBuZXV0cmFsIGNoYXJnZSBhcmUgbmV1dHJvbnMu
Cg==[Qq]
[q]The charge on a neutron is
[c]cG9zaXRpdmU=[Qq]
[c]bmVnYXRpdmU=[Qq]
[c]bmV1dH JhbA==[Qq]
[f]Tm8uIE5ldXRyb25zIGhhdmUgbm8gY2hhcmdlIChtYWtpbmcgdGhlbSBuZXV0cmFsKS4gUHJvdG9uc8KgaGF2ZSBhIHBvc2l0aXZlIGNoYXJnZS4=[Qq]
[f]Tm8uIE5ldXRyb25zIGhhdmUgbm8gY2hhcmdlIChtYWtpbmcgdGhlbSBuZXV0cmFsKS4gVGhlIHBhcnRpY2xlcyB0aGF0IGhhdmUgYSBuZWdhdGl2ZSBjaGFyZ2UgYXJlIGVsZWN0cm9ucy4=[Qq]
[f]RXhjZWxsZW50LiBOZXV0cm9ucyBoYXZlIG5vIGNoYXJnZSAobWFraW5nIHRoZW0gbmV1dHJhbCku
Cg==[Qq]
[q]
The region where electrons are found has three names: energy level, orbit, or shell. Note that the term “orbital,” used in the accompanying video, has a slightly different meaning.
In an uncharged atom (and we’ll talk about how an entire atom can come to have a charge later), the number of protons is equal to the number of electrons. The number of neutrons is more variable (and we’ll talk about that later, too).
True or false: electrons are found in the atom’s nucleus.
[c]VHJ1ZQ==[Qq]
[c]RmFs c2U=[Qq]
[f]Tm8uIFRoZSBzdGF0ZW1lbnQgaXMgZmFsc2UuIEVsZWN0cm9ucyBhcmUgZm91bmQgb3V0c2lkZSB0aGUgbnVjbGV1cywgaW4gd2hhdCBhcmUgY2FsbGVkIGVuZXJneSBsZXZlbHMsIG9yIHNoZWxscy4=[Qq]
[f]RXhjZWxsZW50LiBUaGF0IHN0YXRlbWVudCBpcyBmYWxzZS7CoEVsZWN0cm9ucyBhcmUgZm91bmQgb3V0c2lkZSB0aGUgbnVjbGV1cywgaW4gd2hhdCBhcmUgY2FsbGVkIGVuZXJneSBsZXZlbHMsIG9yIHNoZWxscy4=[Qq]
[q labels = “top”] 3. Atoms, elements, and chemical symbols
Atoms that have the same number of protons and electrons belong to the same element. The atoms of the same element have ____________________________, meaning that they interact with other atoms in the same way. You’ve probably heard about elements such as oxygen, iron, carbon, and uranium.
90 elements occur naturally on Earth, and about twenty more have been artificially created in laboratories. Each element is represented by a one or two letter chemical __________. “H,” for example, is the symbol for hydrogen. “He” is the symbol for Helium. “C” stands for ___________, “N” for Nitrogen, and “O” for oxygen. However, it’s not always the first letter or two of the element’s English name that leads to its symbol. “Au” is the symbol for Gold, “Ag” for Silver, and “Fe” for iron.
[l]Carbon
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]identical chemical properties
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]symbol
[fx] No. Please try again.
[f*] Excellent!
[x]
[restart]
[/qwiz]
2. What You Need to Know (for now) about the Periodic Table of the Elements
The Periodic Table of the Elements organizes all of the elements based on their number of protons and their chemical properties. Each element gets its own square which includes information about that element. That information includes that element’s
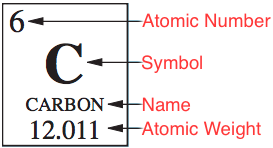
- Atomic number: the number of protons in the atoms of the element.
- Chemical symbol
- Name
- Atomic weight: the sum of the mass of the protons plus the mass of the average number of neutrons.
The most important thing about the Periodic Table is how it’s organized. Here’s what biology students need to know.
- The number of protons in each element increases from left to right in each horizontal row. For example, find the second row in the periodic table (which begins with Li, for Lithium). Lithium has 3 protons. The next element, Beryllium (Be) has 4 protons. The next one, Boron (its chemical symbol is B: you have to jump across a gap to find it) has 5 protons.
- Each vertical column represents a family of elements. The elements in each family have similar chemical properties because the arrangement of their outermost electrons is similar (something you’ll learn about in the next few tutorials). In other words, Lithium and the elements directly below it (starting with Na, or Sodium), are in the same family. They’re all metals that are very reactive (are quick to interact with other elements).
To understand biology, you only need to be familiar with a small number of elements (about a dozen). We’ll meet them in upcoming tutorials.
3. Checking Understanding: The Periodic Table
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Periodic Table Quiz (M3)”]
[h]Checking Understanding: The Periodic Table
[i]Feel free to LOOK at the periodic table provided above to answer the questions that follow.
[q]Find Fe, the symbol for iron. It’s in the fourth row down, and it’s element 26. Which of the following elements would you expect to be most similar to iron in terms of chemical properties?
[c]TW4gKE1hZ25lc2l1bSwgZWxlbWVudCAyNSk=[Qq]
[c]UnUgKFJ1dGhlbml1bS wgZWxlbWVudCA0NCk=[Qq]
[c]Q28gKENvYmFsdCAoZWxlbWVudCAyNyk=[Qq]
[f]Tm8uIE1uIChNYWduZXNpdW0pIGlzIGp1c3QgdG8gdGhlIGxlZnQgb2YgaXJvbi4gSXQmIzgyMTc7cyBpbiB0aGUgc2FtZSByb3csIGJ1dCB0aGF0IGRvZXNuJiM4MjE3O3QgbWFrZSBpdHMgcHJvcGVydGllcyBzaW1pbGFyLiBZb3Ugd2FudCB0byBmaW5kIGFuIGVsZW1lbnQgdGhhdCYjODIxNztzIGluIHRoZSBzYW1lIGNvbHVtbiBiZWNhdXNlIA==ZWxlbWVudHMgaW4gdGhlIHNhbWUgY29sdW1uIGhhdmUgc2ltaWxhciBjaGVtaWNhbCBwcm9wZXJ0aWVzLsKg[Qq]
[f]WWVzLiBSdSAoUnV0aGVuaXVtLCBlbGVtZW50IDQ0KSBpcyBqdXN0IGJlbG93IGlyb24gKEZlKS4gQmVjYXVzZSB0aGV5JiM4MjE3O3JlIGluIHRoZSBzYW1lIGNvbHVtbiwgb3IgZmFtaWx5LCBJcm9uIGFuZCBSdXRoZW5pdW0gd2lsbCBoYXZlIHNpbWlsYXIgY2hlbWljYWwgcHJvcGVydGllcy4=[Qq]
[f]Tm8uIENvIChDb2JhbHQpIGlzIGp1c3QgdG8gdGhlIHJpZ2h0IG9mIGlyb24uIEl0JiM4MjE3O3MgaW4gdGhlIHNhbWUgcm93LCBidXQgdGhhdCBkb2VzbiYjODIxNzt0IG1ha2UgaXRzIHByb3BlcnRpZXMgc2ltaWxhci4gWW91IHdhbnQgdG8gZmluZCBhbiBlbGVtZW50IHRoYXQmIzgyMTc7cyBpbiB0aGUgc2FtZSBjb2x1bW4gYmVjYXVzZSA=ZWxlbWVudHMgaW4gdGhlIHNhbWUgY29sdW1uIGhhdmUgc2ltaWxhciBjaGVtaWNhbCBwcm9wZXJ0aWVzLsKg[Qq]
[q]Find nitrogen (N). It’s in the second row of the periodic table. How many protons does it have?
[c]Nw ==[Qq]
[c]MTQ=[Qq]
[f]WWVzLiBOaXRyb2dlbiYjODIxNztzIGF0b21pYyBudW1iZXIgaXMgc2V2ZW4sIHdoaWNoIG1lYW5zIHRoYXQgaXQgaGFzIHNldmVuIHByb3RvbnMu[Qq]
[f]Tm8uIExvb2sgYXQgdGhlIG51bWJlciBvbiB0aGUgdG9wIGxlZnQgdG8gc2VlIHRoZSBhdG9taWMgbnVtYmVyICh3aGljaCBpcyB0aGUgbnVtYmVyIG9mIHByb3RvbnMpLiBUaGXCoG51bWJlciAxNCBhdCB0aGUgYm90dG9tIG9mIE5pdHJvZ2VuJiM4MjE3O3Mgc3F1YXJlwqByZWZlcnMgdG8gYSBkaWZmZXJlbnQgcHJvcGVydHkgb2Ygbml0cm9nZW4gKHdoaWNoIHdlJiM4MjE3O3JlIG5vdCBnb2luZyB0byB3b3JyeSBhYm91dCByaWdodCBub3cpLg==[Qq]
[q]As you move to the right in each row, the number of protons in each element
[c]aW5jcm Vhc2Vz[Qq]
[c]ZGVjcmVhc2Vz[Qq]
[c]c3RheXMgdGhlIHNhbWU=[Qq]
[f]Q29ycmVjdC4gQXMgeW91IG1vdmUgdG8gdGhlIHJpZ2h0IGluIGVhY2ggcm93LCB5b3Ugc2VlIHRoZSBhdG9taWMgbnVtYmVyIGdvaW5nIHVwLCB3aGljaCBtZWFucyB0aGF0IHRoZSBudW1iZXIgb2YgcHJvdG9ucyBpcyBpbmNyZWFzaW5nLg==[Qq]
[f]Tm8uIEFzIHlvdSBtb3ZlIHRvIHRoZSByaWdodCBpbiBlYWNoIHJvdywgeW91IHNlZSB0aGUgYXRvbWljIG51bWJlciBnb2luZyB1cCwgd2hpY2ggbWVhbnMgdGhhdCB0aGUgbnVtYmVyIG9mIHByb3RvbnMgaXMgaW5jcmVhc2luZw==Lg==[Qq]
[f]Tm8uIEFzIHlvdSBtb3ZlIHRvIHRoZSByaWdodCBpbiBlYWNoIHJvdywgeW91IHNlZSB0aGUgYXRvbWljIG51bWJlciBnb2luZyB1cCwgd2hpY2ggbWVhbnMgdGhhdCB0aGUgbnVtYmVyIG9mIHByb3RvbnMgaXMgaW5jcmVhc2luZw==Lg==[Qq]
[q]An element’s atomic number indicates its
[c]bnVtYmVyIG9mIGVsZWN0cm9ucw==[Qq]
[c]bnVtYmVyIG9mIG5ldXRyb25z[Qq]
[c]bnVtYmVyIG9m IHByb3RvbnM=[Qq]
[f]Tm8uIFRoZSBhdG9taWMgbnVtYmVyIGluZGljYXRlcyB0aGUgbnVtYmVyIG9mIHByb3RvbnMu[Qq]
[f]Tm8uIFRoZSBhdG9taWMgbnVtYmVyIGluZGljYXRlcyB0aGUgbnVtYmVyIG9mIHByb3RvbnM=[Qq]
[f]WWVzLiBUaGUgYXRvbWljIG51bWJlciBpbmRpY2F0ZXMgdGhlIG51bWJlciBvZiBwcm90b25z[Qq]
[x]
[restart]
[/qwiz]
4. Summary: Eight Things to Know About Atoms, Elements, and the Periodic Table
- Atoms are the building blocks of matter
- Atoms have a central nucleus, which contains most of the atom’s mass
- The nucleus contains protons, which are positively charged; and neutrons, which have no charge.
- Negatively charged electrons are found in energy levels or shells outside the nucleus.
- In an uncharged atom, the number of protons equals the number of electrons.
- An element is a substance composed of only one type of atom.
- The Periodic Table of the Elements organizes all of the elements based on their number of protons, and their chemical properties.
- Each element is represented by a one or two-letter chemical symbol.
5. Review and Practice
To make sure that you’ve mastered all of these concepts and terms, try these flashcards:
[qdeck style=”border: 2px solid black; ” random = “true” qrecord_id=”sciencemusicvideosMeister1961-Atomic Structure Flashcards (M3)”]
[h] Flashcards: Atomic Structure
[i] Instructions
- Click ‘Check Answer’ to see the answer to each card.
- If you know it, click ‘Got it.”
- If you don’t know it as well as you’d like, click ‘Need more practice,’ and that card will go to the bottom of the deck so you can practice it again.
- The best practice for learning is to be very strict with yourself. Only click got it if you completely understand the contents of that card.
[!] Card 1+++++++++++++++++++++ [/!]
[q] The building blocks of matter are __________.
[textentry]
[a] The building blocks of matter are atoms.
[!] Card 2+++++++++++++++++++++ [/!]
[q] Most of the mass of the atom is in its ___________.
[textentry]
[a] Most of the mass of the atom is in its nucleus.
[!] Card 3+++++++++++++++++++++ [/!]
[q] Protons and neutrons are found in the __________.
[textentry]
[a] Protons and neutrons are found in the nucleus.
[!] Card 4+++++++++++++++++++++ [/!]
[q] The electrical charge on a proton is __________.
[textentry]
[a] The electrical charge on a proton is positive.
[!] Card 5+++++++++++++++++++++ [/!]
[q] The electrical charge on a neutron is __________.
[textentry]
[a] The electrical charge on a neutron is neutral (no charge).
[!] Card 6+++++++++++++++++++++ [/!]
[q] Negatively charged ____________ are found outside the nucleus.
[textentry]
[a] Negatively charged electrons are found outside the nucleus.
[!] Card 7+++++++++++++++++++++ [/!]
[q] In this diagram, the nucleus is shown at letter [textentry]
[a] In the diagram, the nucleus is indicated by the area indicated by the letter “B.”
[!] Card 8+++++++++++++++++++++ [/!]
[q] In this diagram, the energy levels are represented by the letter [textentry]
[a]In this diagram, the energy levels are represented by letter “A”
[!] Card 9+++++++++++++++++++++ [/!]
[q] In this diagram, the letter ____ is pointing to a neutron. [textentry]
[a] In the diagram, the letter “E” is pointing to a neutron.
[!] Card 10+++++++++++++++++++++ [/!]
[q] In this diagram, the letter ___ is pointing to a proton. [textentry]
[a] In the diagram, the letter “D” is pointing to a proton.
[!] Card 11+++++++++++++++++++++ [/!]
[q] In this diagram, the letter ____ is pointing to an electron. [textentry]
[a] In the diagram, the letter “C” is pointing to an electron.
[!] Card 12+++++++++++++++++++++ [/!]
[q] True or False: In uncharged atoms of the same element, the number of protons is equal to the number of electrons.
[textentry]
[a] The statement is true: In uncharged atoms of the same element, the number of protons is equal to the number of electrons.
[!] Card 13+++++++++++++++++++++ [/!]
[q] True or False: In atoms of the same element, the number of neutrons is equal to the number of protons
[textentry]
[a] The statement was false: The number of neutrons is usually close to the number of protons, but not necessarily the same. In addition, even in atoms of the same element, the number of neutrons can differ.
[!] Card 14+++++++++++++++++++++ [/!]
[q] A substance composed of only one type of atom is a(n) _______.
[textentry]
[a] A substance composed of only one type of atom is an element.
[!] Card 15+++++++++++++++++++++ [/!]
[q] The _________________________ organizes all of the elements based on their number of protons, and their chemical properties
[textentry]
[a] The Periodic Table organizes all of the elements based on their number of protons, and their chemical properties.
[!] Card 16+++++++++++++++++++++ [/!]
[q] Each element is represented by a one or two letter_______.
[textentry]
[a] Each element is represented by a one or two-letter symbol.
[x]If you want more practice with these terms or concepts, please press the restart button below.
[restart]
[/qdeck]
6. Next steps
If you need more practice, please scroll up to the top and work through this tutorial again. Otherwise, continue to Drawing Atoms (the next chemistry tutorial).
