Page outline
- Introduction
- Specificity and Clonal Selection
- Quiz: Antibody Specificity and Clonal Selection
- Antibody Structure and Generating Antibody Diversity
- Quiz: Antibody Diversity and Generating Antibody Diversity
1. Introduction: Review and Preview
Let’s start with a few questions. Don’t worry if you don’t get everything right the first time through!
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Specific Immune System Overview (I.R.)”]
[h]Immune System: Review and Preview
[q] What do all the organisms below have in common?
[c]IFNob3cgbWUgdG hlIGFuc3dlcg==[Qq]
[f]IFRoZXkmIzgyMTc7cmUgYWxsIHZlcnRlYnJhdGVzOiB0aGUgb25seSBjbGFkZSBvZiBvcmdhbmlzbXMgd2l0aCBhIA==c3BlY2lmaWMgaW1tdW5lIHJlc3BvbnNlLg==[Qq]
[q labels = “top”]In the previous tutorial, we examined the first two layers of the body’s defenses against infectious pathogens:
- __________ defenses: the skin and __________ membranes
- _____________ responses, which included
- Cellular defenses: __________ and natural __________ cells
- Molecular defenses: ____________ and interferon
- Tissue-level defenses: ______________.
[l]Barrier
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]complement
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]inflammation
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]mucous
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]killer
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]Non–specific
[fx] No. Please try again.
[f*] Good!
[l]phagocytes
[fx] No. Please try again.
[f*] Great!
[q labels = “top”]In this tutorial, we’ll focus on the ___________ defenses. These include
- ____________: proteins secreted by B cells that bind with specific molecules on the __________ of a pathogen. This binding, as we’ll see, can neutralize the pathogen directly, or help phagocytic cells destroy the pathogen.
- ______________ that seek out and destroy tissue cells that have been infected by _________. While this sounds a lot like the natural killer cells we met in the last tutorial, we’ll see below how killer T cells have ___________ that let them hone in on infected cells with lethal efficiency.
Finally, we’ll learn about how, through the development of ___________ cells, the specific responses enable us to remember some of the pathogens we vanquished in previous infections, and to fight them off so effectively on subsequent exposures that we don’t even realize that we’ve been _________.
[l]Antibodies
[fx] No. Please try again.
[f*] Correct!
[l]infected
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]killer T cells
[fx] No. Please try again.
[f*] Great!
[l]memory
[fx] No. Please try again.
[f*] Great!
[l]receptors
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]specific
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]surface
[fx] No. Please try again.
[f*] Good!
[l]viruses
[fx] No. Please try again.
[f*] Good!
[/qwiz]
My Immune System Part 2: Specific (Adaptive) Immunity music video covers the material we’ll study below. Based on your learning preferences, you might want to view it before or after studying the material below.
2. Specificity and Clonal Selection
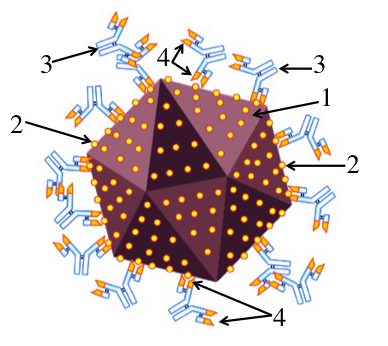
When you’re infected with a virus (“1,” at left), your immune system releases proteins (“3”) that can bind with specific molecules (“2”) on the virus’s surface. These secreted proteins are called antibodies, and by binding with the virus they neutralize it (by preventing the virus from binding with and infecting your cells).
A key thing to note is the specificity of the match between the antibody’s binding site (shown at “4”) and the virus’s surface molecule. In reality, the surface molecule has a very complex three dimensional shape, but I’ve represented it as a circle. In this representation, if the antibody’s binding site had right angles, it wouldn’t bind with this virus, and wouldn’t be able to neutralize it. That’s what we mean by specificity.
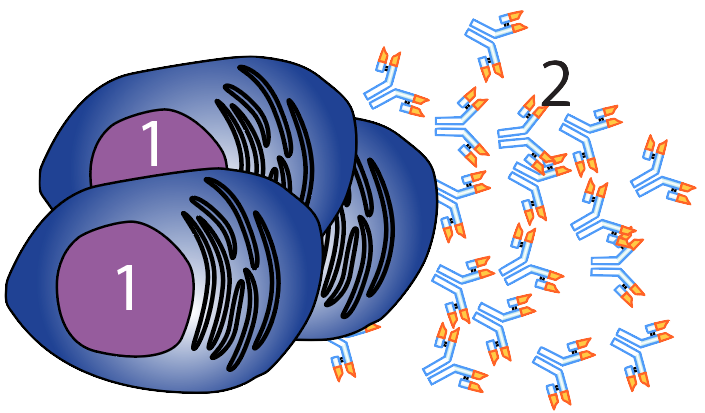
The cells that make antibodies are called B cells. At right, you can see a group of mature B cells (called plasma cells, at “1”) releasing antibodies (2) into the bloodstream. It’s frequently (and incorrectly) said that the immune system will customize a response to a specific pathogen. You might imagine, for example, that your immune system perceives an infection, sizes up the pathogen’s surface molecules, and then, in the same way as you might send a code to a 3-D printer that would print out a piece of plastic with a shape to meet your design, the immune system will churn out an antibody that fits with an invader’s surface molecules. But it’s not like that at all. In fact, the process is much more like natural selection, in which random generation of variation (through mutation and recombination) creates variant organisms with adaptations that get selected by the environment.
This misconception that the immune system tailor-makes a response to each pathogen has been so widespread among my students that I want to spend a bit more time demolishing it before we go on. Let’s think about what it would be like to buy a pair of pants that are tailor-made. Down the street from my house there’s a dry-cleaning shop that employs a tailor. I could walk to the shop, have the tailor measure my legs and waist, show me a choice a fabrics and then she could tailor-make a pair of pants to suit my body’s dimensions. But I have never bought a pair of pants that way. Instead, what I do is go to a local department store, where I see something like what’s shown below and to the right.

The pants are there, in all their variety: colors, cuts, and sizes. I find my size in one of the piles, and head for the cash register.
That’s how I want to encourage you to think of the immune system. The pants are receptors on the B and T cells (a second arm of the immune response) that fight off invaders. These cells, with their various receptors, are pre-made. They’re there before the infection. And in the same way that in natural selection, diversity pre-exists in a generation of offspring that faces various challenges from the environment, the specific immune response is based on pre-existing variation in the B and T cells that wait to face various challenges from pathogens in the environment. But instead of natural selection, what we have here is clonal selection. Here’s an overview of how it works.
Imagine that a there’s a group of B cells in a 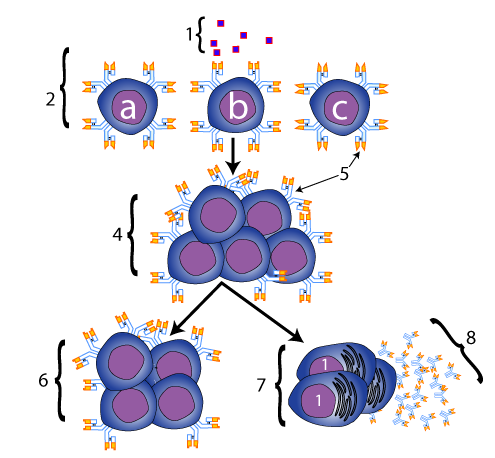 lymph node. We’ll look a bit at the anatomy later, but we often (mistakenly) call lymph nodes “glands,” as when we say that we have an infection and we have “swollen glands.” You know that feeling that you can get in your neck when this happens, so imagine that.
lymph node. We’ll look a bit at the anatomy later, but we often (mistakenly) call lymph nodes “glands,” as when we say that we have an infection and we have “swollen glands.” You know that feeling that you can get in your neck when this happens, so imagine that.
These B cells, shown at “2” at left, aren’t identical. That’s the point. They have various receptors, which are represented by “5” in the diagram at left. Cells “a,” “b,” and “c” all have different receptors.
Now, imagine that your body gets attacked by an invading pathogen. On the surface of the pathogen is a specific molecule, represented by “1.” At this point, we’re going to give this type of molecule, which can elicit an immune response, a name. It’s an antigen. The word antigen, first used at the start of the 1900s, means “antibody generator.” Of the three B cells that we’re considering, only cell “b” has a receptor that can bind with this antigen. That’s because the receptor’s shape is complementary to the shape of the antigen, in the same way that an anticodon is complementary to a codon, or an enzyme is complementary to its substrate. This binding activates the B cell, which creates a clone of B cells (at “4”), all with the same receptor (note again the parallel with natural selection). This clone then matures into two types of descendants. The first is shown below the arrow that goes to the right: these are plasma cells, (shown at “7”), which secrete antibodies (“8”) into the bloodstream. The other descendant is a clone of memory cells (shown at “6”). These cells wait around for another infection, ready to spring into action. These memory cells are the basis of immunity.
3. Antibody Specificity and Clonal Selection: Checking Understanding
[qwiz use_dataset=”SMV_antibody specificity and clonal selection” dataset_intro=”false” random = “true” qrecord_id=”sciencemusicvideosMeister1961-Antibody Specificity and Clonal Selection (Immune Sys. CFU)”]
[h]Antibody Specificity and Clonal Selection
[i]
[/qwiz]
4. Antibody Structure and Generating Antibody Diversity
Let’s look a bit closer at antibodies. As we saw above, antibodies (“2” at left) are proteins secreted by differentiated B cells called plasma cells (“1” at left). Antibodies bind with an antigen. That binding either directly neutralizes the pathogen, or assists other parts of the immune system (the phagocytic cells or complement proteins that we met in the previous tutorial) in neutralizing the pathogen.
proteins secreted by differentiated B cells called plasma cells (“1” at left). Antibodies bind with an antigen. That binding either directly neutralizes the pathogen, or assists other parts of the immune system (the phagocytic cells or complement proteins that we met in the previous tutorial) in neutralizing the pathogen.
For the purposes of an AP Biology course, you can think of antibodies as secreted B cell receptors. So, let’s learn about both.
The image below shows a B-cell receptor in more detail (it’s the entire structure indicated by bracket “2,” below). In this image, “1” represents the B cell, with its membrane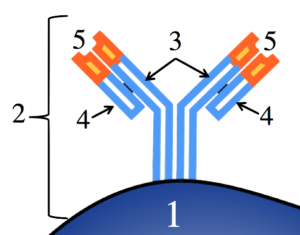 indicated by the black border. The antibody is a quaternary-level protein, consisting of four polypeptide chains. Two of these (“3”) are called heavy chains. The base of these heavy chains can be thought of as anchors that embed the antibody in the B cell’s membrane.
indicated by the black border. The antibody is a quaternary-level protein, consisting of four polypeptide chains. Two of these (“3”) are called heavy chains. The base of these heavy chains can be thought of as anchors that embed the antibody in the B cell’s membrane.
The two heavy chains form a kind of “Y.” Connected to the outer arms of the “Y” are two lighter polypeptide chains (“4”). The chains are connected by disulfide bridges, a type of covalent bond within proteins, so the connection is quite strong.
While the part shown in light blue (the lower part of the heavy and light chains) is constant to all B cell receptors, the tip of the chain, shown at 5, varies from B cell to B cell.
This region is the antigen binding site, and to get an idea of its variety, we need to move away from this cartoon version, and focus on something more realistic (though still a long way from reality).
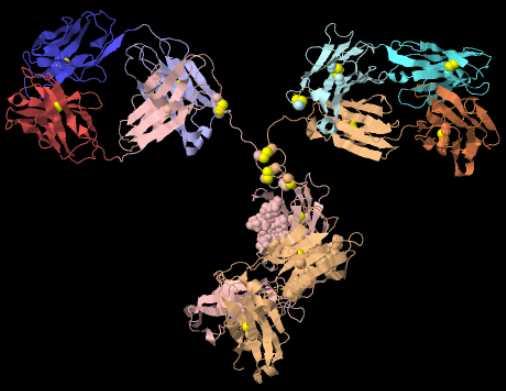
In the diagram to your left, focus on the complexity of the shape at the tips of the outer arms. If you think back to what you’ve learned about protein structure, you’ll notice, within the entire antibody, hairpin turns, folded regions (pleated sheets), and a few corkscrews (alpha helices). The key point is that any variation in amino acid sequence will alter the structure. The tips of your B cell receptors have billions of possible shapes, and these shapes pre-adapt your immune system for interacting with (and ultimately fighting off) an equal number of pathogens.
Immediately above, I mentioned that any variation in amino acid sequence will lead to variation in the shape of an antibody’s antigen binding site. Let’s think genetically for a minute. Amino acid sequence is genetically determined. Remember the central dogma of molecular genetics: DNA makes RNA makes protein. So, if there are millions of different antibodies, there must be millions of different amino acid sequences, which means that we must have millions of different genes, right? WRONG! Like most vertebrate animals, we humans only have about 20,000 genes. So how do we create millions of antibodies?
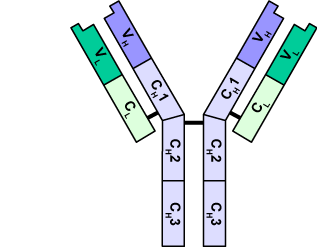 Let’s take another look at an antibody. In the diagram at right, you can see that both the light chains and heavy chains are stitched together. Within the primary structure of each polypeptide chain there are distinct subregions. The light chain consists of a constant sequence of amino acids (CL), connected to a variable region (VL). What that means is that in every antibody of this type, the first sequence of amino acids (CL) is identical, but the second sequence will vary from one antibody to the next. The same is true of the heavy chain. How much variation are we talking about? While the details are complicated (and worth a Nobel Prize for Susumu Tonegawa in 1987: click here to read his acceptance speech), the outcome says it all: the number of antibodies that human beings (and all of our vertebrate cousins) can make is cited as anywhere from 10 billion (wikipedia) to 1 trillion (Molecular Biology of the Cell, 4th edition). Let’s take a brief look at the structure of antibody genes so that we can have an idea about how this diversity is generated.
Let’s take another look at an antibody. In the diagram at right, you can see that both the light chains and heavy chains are stitched together. Within the primary structure of each polypeptide chain there are distinct subregions. The light chain consists of a constant sequence of amino acids (CL), connected to a variable region (VL). What that means is that in every antibody of this type, the first sequence of amino acids (CL) is identical, but the second sequence will vary from one antibody to the next. The same is true of the heavy chain. How much variation are we talking about? While the details are complicated (and worth a Nobel Prize for Susumu Tonegawa in 1987: click here to read his acceptance speech), the outcome says it all: the number of antibodies that human beings (and all of our vertebrate cousins) can make is cited as anywhere from 10 billion (wikipedia) to 1 trillion (Molecular Biology of the Cell, 4th edition). Let’s take a brief look at the structure of antibody genes so that we can have an idea about how this diversity is generated.
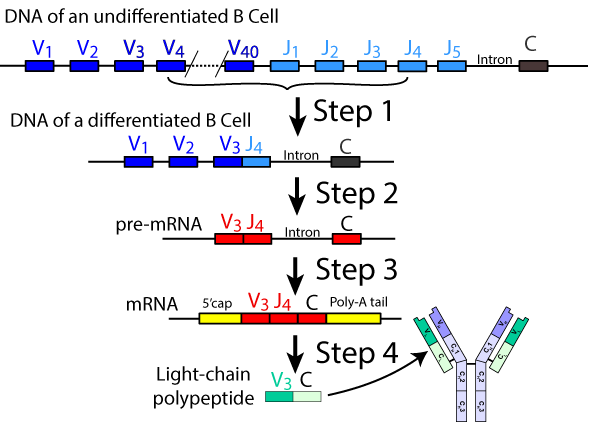
What’s shown at left is a very simplified view depicting the sequence of events involved in generating diversity in the light polypeptide chain. These genes are found on human chromosomes 2 and 22. As you can see, the original DNA for these genes includes 40 variable segments (V1 through V40), 5 joining segments (J1 through 5), introns, and a Constant region (C).
- Step 1: As the B cell differentiates (a process that occurs within our bone marrow), a team of enzymes that includes nucleases (which cut the DNA), recombinases (which recombine DNA segments), and ligase (which stitches DNA segments together) randomly snips out one of the variable sequences and one of the joining segments, and splices them together (see above). The genes for the other V and J segments are permanently edited out of this cell line. Note that this is a type of DNA editing that, in your studies of biology, you probably haven’t seen before. In everything you’ve seen about gene expression, DNA is always safely maintained in the nuclear vault. Note that here, the DNA in the nucleus of this developing B cell has been permanently modified, with genes lost.
- Steps two and three should be familiar to you from your previous studies of transcription and RNA processing. In step 2, RNA polymerase transcribes the DNA into pre-mRNA. In step 3, splicing enzymes cut out the intron, splice the exons together, and add a protective poly A tail and a 5′ cap.
- Step 4 is translation into the light chain polypeptide, which becomes incorporated into the finished antibody/receptor.
The variation described above is enhanced by two other features:
- In the heavy chain, there is an additional class of genes called diversity genes. Random recombination that involves splicing these genes together with a similar number of variable and joining segment genes makes the heavy chain even more variable than the lighter chain.
- As the B cell continues to differentiate, a process called somatic hypermutation increases the mutation rate of antibody region genes. This generates additional diversity in antigen binding sites.
What’s the takeaway from all this? Just remember that antibody diversity in humans and our vertebrate cousins is
- vast (billions of receptors and antibodies waiting for pathogens)
- pre-existing (like the pants in a department store).
- created through recombination and mutation of genes for antibody regions that occurs as B cells develop.
Finally, keep in mind that while the details what happens with T cell receptors is different, the principles are the same, with the result that T cell receptors have similar degrees of diversity to antibodies/B cell receptors.
Got it? Try answering a few questions about antibody diversity.
5. Antibody Structure and Antibody Diversity: Checking Understanding
[qwiz random = “true” use_dataset=”SMV Antibody structure and antibody diversity” dataset_intro=”false” qrecord_id=”sciencemusicvideosMeister1961-Antibody Structure and Diversity, Immune Sys. CFU”]
[h]Antibody Structure and Antibody Diversity
[i]
[/qwiz]
Next Steps
- Immune System 3: The Humoral Response
- Return to the Immune System Main Menu
