Looking for a student learning guide? You’ll find a link on the main menu for your course. Use the “Courses” menu above.
Introduction
In the previous tutorial, we looked at the chemistry of water. We saw how water’s polar structure allows water molecules to form hydrogen bonds with other water molecules. In this tutorial, we’ll do a virtual lab that lets us look at water’s unique properties: properties that result from hydrogen bonding.
If it suits your learning style, you might want to start by watching the video below.
1. A (virtual) lab comparing water and alcohol: What you’ll need
To get a sense of the properties of water, we’re going to do a virtual lab comparing water to alcohol. Since real science is always preferable to virtual science, I want to encourage you to get the supplies for this lab and to independently try the activities. Alternatively, your teacher might be taking you through this lab in a classroom setting.
If you’re working independently, here’s what you’ll need:
- Tap water
 A small bottle of Isopropyl alcohol. This kind of alcohol is available at almost any supermarket or pharmacy. 91% or higher is the best concentration for this lab.
A small bottle of Isopropyl alcohol. This kind of alcohol is available at almost any supermarket or pharmacy. 91% or higher is the best concentration for this lab.
- SAFETY NOTE: isopropyl alcohol is poisonous. Don’t drink it. Wear goggles or safety glasses to protect your eyes.
- Also, if you can’t get isopropyl alcohol, but other types of alcohol are available (such as ethyl alcohol), the lab should work perfectly well.
- Two eye droppers or plastic pipettes. If you’re using pipettes, label them, and put one in the water, and the other in the alcohol. If you’re using droppers, fill one with water, and one with alcohol.
- A few small beakers, glasses, or cups.
- Two small paper clips
- Two pennies (or two small squares of wax paper if you can’t get a penny)
- Two teaspoons to do some stirring
- Table salt (about two teaspoons).
2. Background: Comparing Water and Isopropyl Alcohol
Based on our last tutorial, you already know a lot about water. Here’s a quick review of water’s key properties.
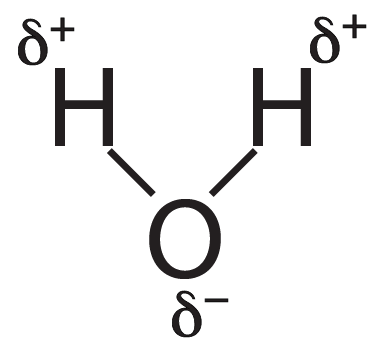
- Water is a polar molecule.
- The oxygen side of the molecule has a partially negative charge (?–)
- The hydrogens, on the other side, have a partially positive charge(?+).
- As a result, water molecules form hydrogen bonds, interacting as shown below.
 |
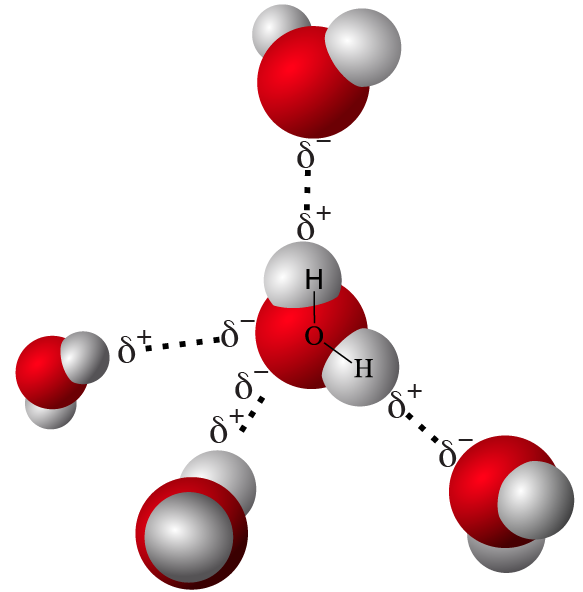 |
| Water: structural formula | Water molecules forming hydrogen bonds |
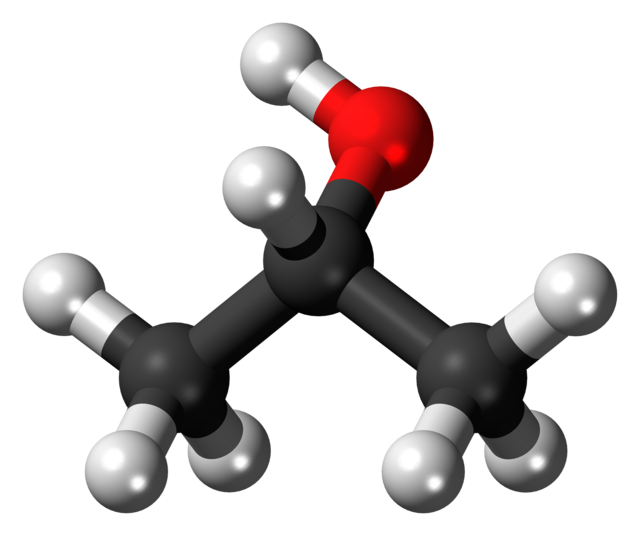
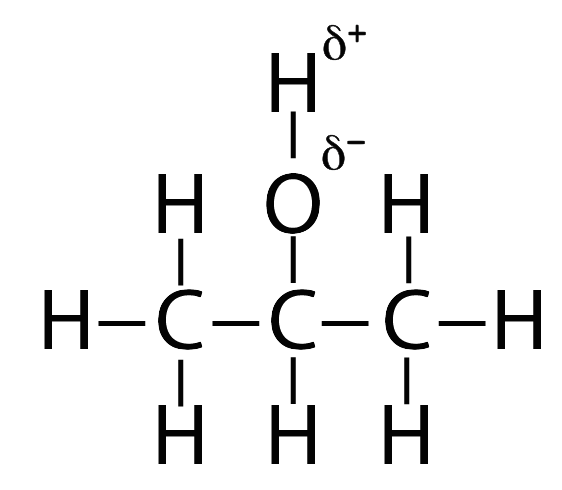
Here’s isopropyl alcohol.
Here’s what you need to know about isopropyl alcohol:
- Most of the isopropyl alcohol (the three carbons and the hydrogens attached to them) is non-polar.
- One part of isopropyl alcohol is slightly polar. It’s the oxygen and hydrogen on top. Notice that in the structural formula on the left, the hydrogen (H) has a ?+ (partial positive charge) next to it, and the oxygen has a ?– (partial negative charge)
So, isopropyl alcohol is slightly polar. But it’s much less polar than water is.
 |
 |
| Water: highly polar | Isopropyl Alcohol: much less polar than water |
Knowing that, let’s do a few experiments where we compare them.
3. Six Observations/Experiments Comparing Water and Alcohol
Please record your predictions for each of the experiments that follow on your student learning guide. You’ll learn a lot more if you make a prediction before clicking “show the answer.”
[qwiz style = ” width: 600px !important; min-height: 400px !important; border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Water v Alcohol Virtual Lab (2.0)”]
[h]Water v. alcohol: Virtual Lab
[q]EXPERIMENT 1: Let’s start by predicting the way that a single drop of water and a single drop of alcohol will look when you put them on a surface like a penny or wax paper. Do the following
- In your notes, draw a picture of how you think a single drop of water on a penny will look, compared with how a single drop of alcohol will look.
- Below your drawing, explain why you think the two will be different.
- Once you’ve made your prediction, do the experiment if you have materials.
- Click “show the answer.”
[c]IMKgc2hvdyB0 aGUgYW5zd2Vy[Qq]
[f]IEFOU1dFUg==
CgoK| [Qq]A single drop of water on a penny | A single drop of alcohol on a penny |
Because the water molecules are polar, they grab onto one another through hydrogen bonds. The mutual attraction of water molecules is called cohesion. Cohesion pulls a drop of water into a spherical shape that allows for the maximum number of bonds. Though gravity is trying to pull the water molecules down, the hydrogen bonds keep the water droplet in a spherical shape. Because alcohol is so much less polar, it can’t resist gravity, and all of the molecules are pulled down into a flat sheet over the penny’s surface.
[q]EXPERIMENT 2: Use what you just observed to predict the following: How many drops of water can you put onto a penny before the water spills over the side? How many drops of alcohol before the alcohol spills over the side? Make a prediction, give it a try, and, most importantly, explain what’s happening.
When you’re ready, click “show the answer” to see the results and verify your prediction.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==| [Qq]Water on a penny | Alcohol on a penny |
Many variables influence the exact number of drops, but you should have been able to pile many more drops of water on the penny than drops of alcohol. Again, this is because the water molecules, which form hydrogen bonds with one another, have much more cohesion than alcohol molecules. Cohesion forces the water to form a sphere, with more and more water molecules filling that sphere (until gravity overcome hydrogen bonding, and water spills out over the side.
With less polarity, the alcohol can’t resist gravity. Its molecules are pulled down into a flat sheet over the penny’s surface that can accommodate many fewer drops than the hydrogen-bond induced sphere that’s on the penny with water.
[q] EXPERIMENT 3: Speed of Evaporation in Water and Alcohol
This video compares the evaporation of a drop of alcohol (left) with a drop of water (right). I used my phone as both a surface and a timer (and then speeded up the video about 60 times, so that each minute on the clock goes by in a second). If you can do the same, go ahead and try it.
Explain why the evaporation times for water and alcohol are so different.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==VGhlIHNwZWNpZmljIHByb3BlcnR5IHRoYXQgd2UmIzgyMTc7cmUgY29tcGFyaW5nIGhlcmUgaXMgdGhlIA==aGVhdCBvZiB2YXBvcml6YXRpb24=OiBpdCYjODIxNztzIHRoZSBhbW91bnQgb2YgaGVhdCB0aGF0JiM4MjE3O3MgcmVxdWlyZWQgdG8gbWFrZSBhIGxpcXVpZCBpbnRvIGEgZ2FzLiBXYXRlciYjODIxNztzIGhlYXQgb2YgdmFwb3JpemF0aW9uIGlzIHZlcnkgaGlnaCBhbmQgbXVjaCBoaWdoZXIgdGhhbiB0aGF0IG9mIGFsY29ob2wuIFRoYXQmIzgyMTc7cyBiZWNhdXNlIHdhdGVyLCBiZWNhdXNlIG9mIGh5ZHJvZ2VuIGJvbmRpbmcsIHJlc2lzdHMgZXZhcG9yYXRpb24uIFRoZSBoeWRyb2dlbiBib25kcyBiZXR3ZWVuIHRoZSB3YXRlciBtb2xlY3VsZXMga2VlcCB0aG9zZSBtb2xlY3VsZXMgZnJvbSBqdW1waW5nIGF3YXkgZnJvbSB0aGUgb3RoZXIgbW9sZWN1bGVzIGluIHRoZSBsaXF1aWQgYW5kIGJlY29taW5nIGEgZ2FzLg==
[Qq]Because alcohol is mostly a nonpolar molecule (with just a bit of polarity) there’s very little attraction between one alcohol molecule and the next. Consequently, when alcohol molecules absorb enough energy from the environment to start moving around quickly, they can easily accelerate to a high enough speed to jump away from the surface of the liquid. No hydrogen bonds are holding them back. As molecule after molecule of alcohol jumps away from the liquid, the volume of the liquid decreases…until all of it has evaporated.
[q]EXPERIMENT 4: Predict how water and alcohol will feel when you place a few drops of each liquid on your skin.
- In your notes, predict how each liquid will feel when placed on your skin. Below your prediction, explain why you think the two will be different.
- If you have the materials, place the same number of drops of alcohol and water on different areas of your skin (for example, different parts of the back of your hand). If you can, work with a partner, and have your partner place the drops on your hand while your eyes are closed. Without looking, can you tell which drop was which? Afterward, click on “show the answer” to see if your results correspond with what I’ve written below.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==QWxjb2hvbCBmZWVscyBtdWNoIGNvb2xlciBvbiB5b3VyIHNraW4gdGhhbiBhIGRyb3Agb2Ygd2F0ZXIuIEFzIHlvdSBtaWdodCBiZSBleHBlY3RpbmcsIHRoZSByZWFzb24gZm9yIHRoZSBkaWZmZXJlbmNlIGlzIGh5ZHJvZ2VuIGJvbmRpbmcsIGFuZCB3YXRlciYjODIxNztzIG11Y2ggaGlnaGVyIGhlYXQgb2YgdmFwb3JpemF0aW9uIHRoYW4gYWxjb2hvbC4gQmVjYXVzZSBvZiBoeWRyb2dlbiBib25kcywgdGhlIHdhdGVyIG1vbGVjdWxlcyBhcmVuJiM4MjE3O3QgZXZhcG9yYXRpbmcuIFRoYXQgbWVhbnMgdGhhdCBhcyB5b3VyIGJvZHkgaGVhdCB3YXJtcyB1cCB0aGUgd2F0ZXIgbW9sZWN1bGVzLCB0aGV5JiM4MjE3O3JlIHdhcm1pbmcgdXAsIHRvby4gVGhlIGFsY29ob2wgbW9sZWN1bGVzLCBieSBjb250cmFzdCwgYXJlbiYjODIxNzt0IGNvbm5lY3RlZCBieSBoeWRyb2dlbiBib25kcy4gQXMgdGhleSBhYnNvcmIgaGVhdCBmcm9tIHlvdXIgc2tpbiwgdGhleSBzdGFydCB0byBtb3ZlIGZhc3RlciBhbmQgZmFzdGVyLiBBcyB0aGV5IHN0YXJ0IHRvIGV2YXBvcmF0ZSwgdGhleSBjYXJyeSB0aGVpciBoZWF0IGVuZXJneSBhd2F5IGZyb20geW91ciBza2luLiBZb3UgcGVyY2VpdmUgdGhhdCBsb3NzIG9mIGhlYXQgZW5lcmd5IGFzIGNvb2xuZXNzLg==[Qq]
[q]EXPERIMENT 5: Now let’s predict how easily salt will dissolve in water and alcohol.
- In your notes, predict whether or not salt will dissolve in water, and whether or not salt will dissolve in an equal amount of alcohol.
- If you have the materials, use two cups.
- place a small amount of water (100 mL) in a cup, and an equal amount of alcohol in a second cup.
- Use one teaspoon to put about 1/2 a teaspoon of salt in the water cup. Stir. Record what happens.
- Using a second teaspoon, put about 1/2 a teaspoon of salt into the alcohol cup. Stir. Record what happens.
- If you don’t have the materials, just click on “show the answer” to see the results and verify your prediction.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==V2hpbGUgc2FsdCBxdWlja2x5IGRpc3NvbHZlcyBpbiB3YXRlciwgaXQgd29uJiM4MjE3O3QgZGlzc29sdmUgaW4gYWxjb2hvbC4=
Cg==[Qq]
Table salt is sodium chloride (NaCl). Sodium chloride is an ionic compound, held together by ionic bonds between positively charged sodium ions and negatively charged chlorine ions. When water and salt are mixed, water’s polarity makes dissolving the salt easy. The positively charged side of water pulls the chlorine ions away from the salt crystal. The negatively charged side of water pulls the sodium ions away from the salt crystal. In the image on the left, developed by Professor Angelos Michaelides, University College, London, you can see this. The green spheres are chlorine; the blue spheres are sodium.
In less polar alcohol, the alcohol molecules have no way to “grab” onto the sodium or chloride ions, which stay connected through ionic bonds, and don’t dissolve.
[q]EXPERIMENT 6: See if you can float a paper clip on the surface of a cup of water. Then see if you can do the same with alcohol. Here’s the procedure. If you don’t have the materials, read the procedure anyway, and try to predict what will happen in each case.
- Fill a cup with water. To make this easy, use a small cup (but one that’s big enough for a paper clip to float on the surface.
- Use a small paper clip. It’s easiest if you bend up a short metal handle from the paper clip so you can place the clip on the water as gently as possible. Bend it like this
- Gently place the paper clip on the surface of the water, then let go.
- Repeat the same step, except with alcohol in a cup instead of water.
[c]wqBzaG93IHRo ZSBhbnN3ZXI=[Qq]
[f]IEFOU1dFUg==
Cg==| [Qq]Paper clip: floating on water | Paper clip: sunk in alcohol |
Here’s what’s happening.
Because water molecules are polar, they connect through hydrogen bonds. So imagine the paper clip floating on a web of water molecules (as shown in this image, which is ridiculously not drawn to scale). As long as the clip doesn’t break the web, it can float on top. The name for this web of water molecules on the surface of a body of water is surface tension.
Alcohol is much less polar than water. Because it’s non-polar, the molecules don’t form hydrogen bonds. Because they don’t form hydrogen bonds, the clips sink through the surface. Essentially, in the alcohol solution, there’s no surface tension (or, at least, not nearly enough to support a paper clip).
[x]
[/qwiz]
4. Additional points about water and hydrogen bonding
In the lab above, we addressed
- Cohesion
- Surface tension
- Heat of vaporization
- Water’s ability to dissolve polar and ionic substances.
Here are a few more AP Bio-related concepts to know about related to water and hydrogen bonding.
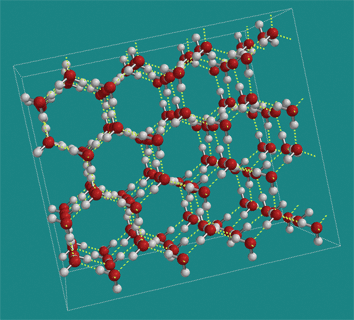
4a. Frozen water has unique properties
Water has a relatively high freezing point. That’s because when water molecules get cool enough, they’ll grab onto one another through hydrogen bonding. Those bonds lock the molecules into the fixed, solid molecular matrix we know as ice. By contrast, isopropyl alcohol forms few hydrogen bonds and has to be cooled to -89 °C to become a solid. Methane (CH4), which is completely nonpolar, has about the same molecular weight as water, but only liquifies at -182°C (and won’t solidify at all).
Water is less dense as a solid than as a liquid. As a result, ice floats on water. This is because when water freezes, the hydrogen bonds generate a crystal matrix in which the molecules are more spread out than when water is in a liquid form. This is a unique property: water might be the only substance whose solid form is less dense than its liquid form.
4b. Adhesion
Hydrogen bonding can lead to adhesion as well as cohesion. Adhesion is the molecular attraction between adjacent surfaces. Through hydrogen bonding, water can bond to other polar substances. This explains phenomena such as capillary action, in which water will flow into a narrow space such as a thin tube, even in opposition to the force of gravity. Adhesion between water molecules and the thin tubes in the stems of plants plays a major role in the process of transpiration, which is how plants move water from their roots up to their leaves.
4c. Specific Heat
Because of hydrogen bonding, water also has a very high specific heat: the amount of heat required to raise one gram of a substance by one degree C. Water has a high specific heat because the hydrogen bonds that form between water molecules constrain their movement. Since temperature is the average kinetic energy of the molecules within an object, that means that as you add heat to a body of water, there’s not a corresponding increase in the molecules’ kinetic energy. As a result, the temperature doesn’t rise. One such watery body that resists wide temperature fluctuations is your body (or the body of any relatively large animal). Hydrogen bonds help keep the temperature within the body of any relatively large organism — on a molecular scale, that means anything the size of an earthworm or larger — relatively constant. It also keeps the Earth’s planetary temperature mild, because the oceans can absorb lots of heat without their temperature rising.
4d. Hydrogen bonding can occur between any two polar molecules.
For example, the two strands of DNA are held together by hydrogen bonds. These bonds are shown at number 6 in the image of DNA on the left below. On the right, you can see a closeup of the bonds between the nitrogenous bases that make up the central core of a DNA molecule. Note that the hydrogen bonds form between oxygen atoms and hydrogen atoms, or between nitrogen atoms and hydrogen atoms.
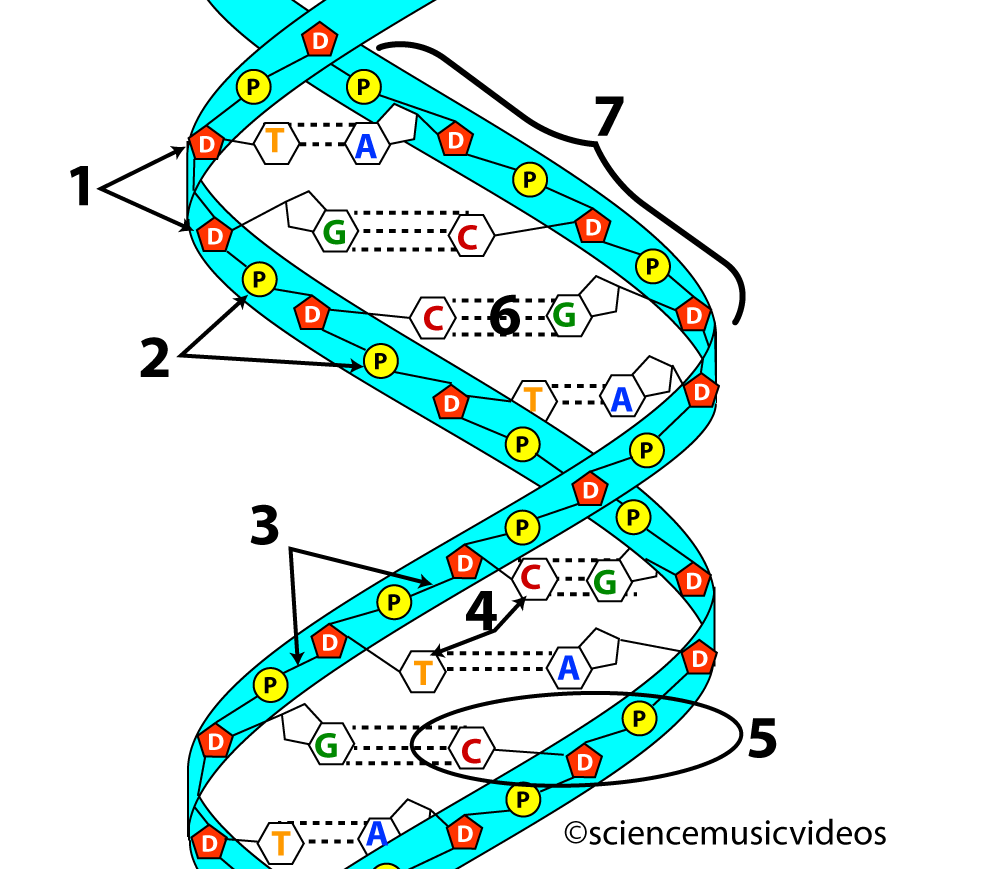 |
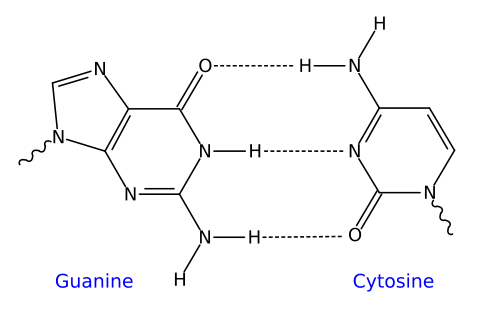 |
5. Video: Water Chemistry and Properties Part 2
The video below shows everything you’ve learned above
6. Checking Understanding: Properties of Water
[qwiz random = “false” qrecord_id=”sciencemusicvideosMeister1961-Properties of Water F-I-B Quiz”]
[h]Properties of Water Quiz
[i]This quiz is a combination of the game “hangman” and a flashcard deck. With each question, you’ll type in the answer. If you get the question wrong, it goes back in the deck for you to repeat it.
[q] Water is a [hangman] molecule. As a result, water molecules form [hangman] bonds with one another.
[c]cG9sYXI=[Qq]
[c]aHlkcm9nZW4=[Qq]
[q]Whereas the [hangman] side of water has a partial positive charge, the [hangman] side of water has a partial negative charge
[c]aHlkcm9nZW4=[Qq]
[c]b3h5Z2Vu[Qq]
[q]Because it has less [hangman] bonding, a drop of alcohol will have a much [hangman] shape than a drop of water. That’s because the bonds between water molecules create a lot of [hangman], a property that’s lacking in alcohol.
[c]aHlkcm9nZW4=[Qq]
[c]ZmxhdHRlcg==[Qq]
[c]Y29oZXNpb24=[Qq]
[q]The amount of heat required to make a liquid into a gas is its heat of [hangman].
[c]dmFwb3JpemF0aW9u[Qq]
[q]Because it forms fewer [hangman] bonds, alcohol has a much [hangman] heat of vaporization than water.
[c]aHlkcm9nZW4=[Qq]
[c]bG93ZXI=[Qq]
[q]Because water is polar, it’s great at dissolving [hangman] substances like salt and just about any [hangman] substance (such as sugar).
[c]aW9uaWM=[Qq]
[c]cG9sYXI=[Qq]
[q]Because of hydrogen bonding, water has a lot of [hangman] [hangman], enabling a paper clip to float on the surface of a body of water.
[c]c3VyZmFjZQ==[Qq]
[c]dGVuc2lvbg==[Qq]
[q]Because of hydrogen bonding, water freezes at a relatively [hangman] temperature. In addition, hydrogen bonding makes ice [hangman] dense than water, causing ice to [hangman].
[c]aGlnaA==[Qq]
[c]bGVzcw==[Qq]
[c]ZmxvYXQ=[Qq]
[q]Whereas [hangman] involves attraction between water molecules (caused by hydrogen bonds), [hangman] involves hydrogen bonds forming between water molecules and another surface (such as the walls of the tubes that conduct water up the stem of a plant.
[c]Y29oZXNpb24=[Qq]
[c]YWRoZXNpb24=[Qq]
[q]The diagram below shows how [hangman] bonds can form between any two [hangman] molecules, and not just between water molecules.
[c]aHlkcm9nZW4=[Qq]
[c]cG9sYXI=[Qq]
[q] [hangman] heat is the amount of heat required to raise one gram of a substance by one degree C. Because of this property, bodies of water resist fluctuations in [hangman]. This has created a moderate climate on Earth owing to the role of the oceans as climate regulators.
[c]c3BlY2lmaWM=[Qq]
[c]dGVtcGVyYXR1cmU=[Qq]
[x][restart]
[/qwiz]
What’s next
- Proceed to Topic 1.1, Part 3: Acids, Bases, and the pH Scale (the next tutorial in AP Bio Unit 1)