1. Osmotic Pressure
The movement of water caused by osmosis generates a force called osmotic pressure. Osmotic pressure explains why our gummy bear expanded. Let’s look at it in a bit more detail.
| Original Situation | After Osmosis |
 |
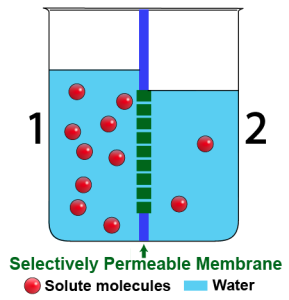 |
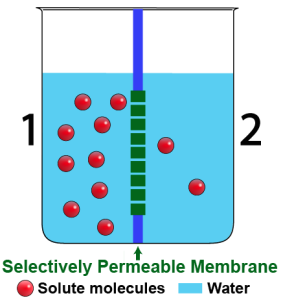
The beakers above show a time sequence. The beaker on the left shows the situation at the start: side 2 is hypotonic to side 1. As a result, water will move, by osmosis, from side 2 into side 1.
Water can freely diffuse across this membrane. However, once the water molecules diffuse across the membrane, they interact with the solute in a way that keeps them from returning. As a result, water molecules leave side 2, enter side 1, and stay there. With more water molecules on side 1, the water level has to rise. With fewer water molecules on side 2, the water level there has to fall. You can see the result in the beaker on the right.
2. Animal Cells and Osmosis
Let’s see how this can affect cells in hypotonic, isotonic, or hypertonic solutions.
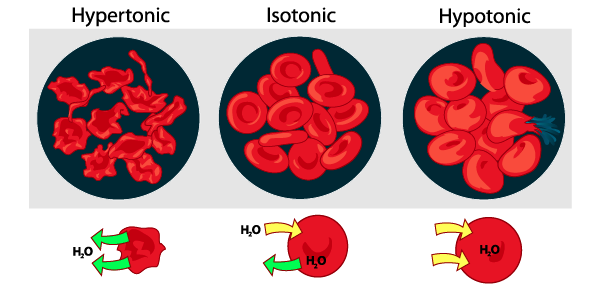
Consider the red blood cells shown above.
The cells in the hypotonic solution (on the right side of the diagram) are expanding and bursting. Why?
These cells were hypertonic to the surrounding solution. Because water flows from hypotonic to hypertonic, water will flow into the cells. This flow of water exerts pressure, much like the force you exert when you blow up a balloon. In the same way that a balloon expands, so will the cell, right up to the point where it bursts. In the same way that air pressure can burst a balloon, osmotic pressure can burst a red blood cell.
By contrast, the cells in the hypertonic solution (the one on the left) are shriveled. Why? Because water flows from hypotonic to hypertonic. If the cells are in a hypertonic solution, water will leave the cells, and flow into the solution. The loss of water causes the cells to shrivel up, just like the loss of water from a grape causes it to become a shriveled raisin. In the same way that loss of air pressure causes a tire to become flat (deflated), loss of osmotic pressure can cause a cell (and living tissues made of cells) to become shriveled.
In an isotonic solution (shown in the middle), water flows equally between the cells and the solution. The cell doesn’t gain water or lose water (because water diffuses into and out of it at equal rates).
3. Plant Cells and Osmosis
Because plant cells have a rigid wall, they respond to osmotic pressure differently.
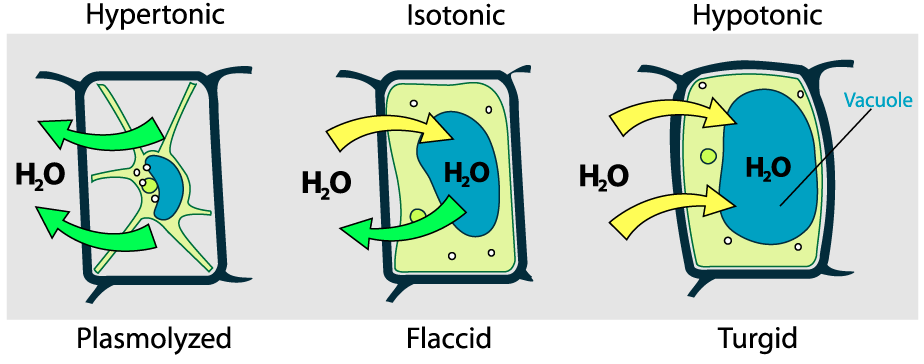
- In a hypertonic solution, a plant cell will lose water. As it does, the central vacuole contracts. This causes the cell membrane to peel away from the wall, a condition called plasmolysis. As a result, the plant as a whole becomes wilted and droopy.
- In a hypotonic solution, water will move into the plant cell. This will cause the central vacuole to expand. As it does, the entire cell will expand (just as the red blood cells did above). However, unlike red blood cells in a hypotonic solution, these plant cells won’t burst. Instead, the membrane will be pushed against the wall, keeping the plant cell solid and firm.
This is a healthy condition for plant cells and plants as a whole. It’s why grocery stores use misters to keep their vegetables covered with water (a hypotonic solution), causing water to move into the veggies to keep them crisp and firm.
 |
 |
| Wilted lettuce, caused by loss of osmotic pressure | A grocery mister. The hypotonic water keeps the veggies crisp. |
4. Osmosis Quiz 1: Diagrams and Vocabulary
[qwiz style = “border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Osmosis Quiz 1 (M8)”]
[h] Osmosis Quiz 1: Diagrams and Vocabulary
[i]This activity tests your understanding of diagrams related to osmosis. Here’s how the quiz works:
- Each question is multiple-choice, but the entire quiz is like a series of flashcards.
- If you get the question right, it comes off the deck.
- If you get the question wrong, it goes to the bottom of the deck, so you can try it again.
[q labels = “top”]
[l]selectively permeable membrane
[fx] No. Please try again.
[f*] Correct!
[l]hypertonic side
[fx] No, thatís not correct. Please try again.
[f*] Good!
[l]hypotonic side
[fx] No. Please try again.
[f*] Good!
[l]solute molecule
[fx] No. Please try again.
[f*] Great!
[l]water
[fx] No. Please try again.
[f*] Great!
[q labels = “top”]A gummy bear is put into water. Gummy bears are full of sugar. Label this osmotic situation.
[l]hypotonic
[fx] No, thatís not correct. Please try again.
[f*] Correct!
[l]hypertonic
[fx] No. Please try again.
[f*] Correct!
[q labels= “top”]Label this osmotic situation.
[l]hypotonic
[fx] No, thatís not correct. Please try again.
[f*] Good!
[l]hypertonic
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]Label this osmotic situation
[l]hypotonic
[fx] No. Please try again.
[f*] Good!
[l]hypertonic
[fx] No, thatís not correct. Please try again.
[f*] Great!
[q labels = “top”]Label the osmotic situation of the cell below, which is 95% water.
[l]hypertonic
[fx] No, thatís not correct. Please try again.
[f*] Correct!
[l]hypotonic
[fx] No, thatís not correct. Please try again.
[f*] Excellent!
[q labels = “top”]Label the osmotic situation of the cell below, which is 98% water.
[l]hypotonic
[fx] No, thatís not correct. Please try again.
[f*] Great!
[l]hypertonic
[fx] No, thatís not correct. Please try again.
[f*] Good!
[q labels = “top”]Label the osmotic situation of the cell below, which is 98% water.
[l]hypertonic
[fx] No, thatís not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No. Please try again.
[f*] Great!
[q labels = “top”]Drag the labels to complete the sentence: the cell is in a(n) ______ environment.
[l]isotonic
[fx] No, thatís not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No. Please try again.
[f*] Great!
[l]hypertonic
[fx] No. Please try again.
[f*] Good!
[q labels = “top”]Drag the labels to complete the sentence: the cell is in a(n) ______ environment.
[l]isotonic
[fx] No, that is not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No, that is not correct. Please try again.
[f*] Excellent!
[l]hypertonic
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]Drag the labels to complete the sentence: the cell is ______ to its environment.
[l]isotonic
[fx] No, that is not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No. Please try again.
[f*] Great!
[l]hypertonic
[fx] No. Please try again.
[f*] Good!
[q labels = “top”]Drag the labels to complete the sentence: the cell is ______ to its environment.
[l]isotonic
[fx] No, that is not correct. Please try again.
[f*] Great!
[l]hypotonic
[fx] No, that is not correct. Please try again.
[f*] Excellent!
[l]hypertonic
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]Drag the labels as if you were completing the sentence “These elodea cells are ____________ to their environment.”
[l]hypotonic
[fx] No. Please try again.
[f*] Correct!
[l]hypertonic
[fx] No. Please try again.
[f*] Good!
[q labels = “top”]Drag the labels as if you were completing the sentence “These elodea cells are in a(n) ____________ environment.”
[l]hypotonic
[fx] No. Please try again.
[f*] Correct!
[l]hypertonic
[fx] No. Please try again.
[f*] Good!
[q]Label the parts of these elodea cells. The cells start in fresh water and then are exposed to salt water.
[l]cell membrane
[fx] No. Please try again.
[f*] Correct!
[l]cell wall
[fx] No. Please try again.
[f*] Excellent!
[l]chloroplast
[fx] No, thatís not correct. Please try again.
[f*] Correct!
[q]The movement of a substance from higher concentration to lower concentration is [hangman],
[c]ZGlmZnVzaW9u[Qq]
[q]The diffusion of water is [hangman]
[c]b3Ntb3Npcw==[Qq]
[q]A mixture in which one thing is dissolved in another is a(n) [hangman].
[c]c29sdXRpb24=[Qq]
[q]In a solution, the thing that does the dissolving is the [hangman]. The thing that gets dissolved is the [hangman].
[c]c29sdmVudA==[Qq]
[c]c29sdXRl[Qq]
[q]In lemonade (that you make from a mix), the lemonade mix is the [hangman]. The water is the [hangman].
[c]c29sdXRl[Qq]
[c]c29sdmVudA==[Qq]
[q]Solution “A” has more dissolved solute than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwZXJ0b25pYw==[Qq]
[q]Solution “A” has less dissolved solute than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwb3Rvbmlj[Qq]
[q]Solution “A” has the same amount of dissolved solute as solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aXNvdG9uaWM=[Qq]
[q]Solution “A” has a higher percentage of water than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwb3Rvbmlj[Qq]
[q]Solution “A” has a lower percentage of water than solution “B.” Solution “A” is [hangman] to solution “B.”
[c]aHlwZXJ0b25pYw==[Qq]
[q]Diffusion is the movement of a substance [hangman] its concentration gradient.
[c]ZG93bg==[Qq]
[q]Osmosis is the diffusion of [hangman].
[c]d2F0ZXI=[Qq]
[q]If solution “A” has [hangman] solute than solution “B,” then solution “A” is hypertonic to solution “B.”
[c]bW9yZQ==[Qq]
[q]If I want to make a solution more hypertonic, I add more [hangman].
[c]c29sdXRl[Qq]
[x]
[restart]
[/qwiz]
5. Osmosis Quiz 2: Multiple Choice
[qwiz style = “border: 3px solid black; ” qrecord_id=”sciencemusicvideosMeister1961-Osmosis Quiz 2 (M8)”]
[h] Quiz: Osmosis
[i]This quiz tests you on your understanding of osmosis, and your ability to explain phenomena like the one below.
[q]A solution that has a higher solute concentration than a solution on the other side of the membrane is ___________
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7IFdoYXQgcHJlZml4IGlzIGFzc29jaWF0ZWQgd2l0aCAmIzgyMjA7bW9yZSYjODIyMTsgb3IgJiM4MjIwO3RvbyBtdWNoLiYjODIyMTs=[Qq]
[f]RXhjZWxsZW50LsKgQSBzb2x1dGlvbiB0aGF0IGhhcyBhIGhpZ2hlciBzb2x1dGUgY29uY2VudHJhdGlvbiB0aGFuIGEgc29sdXRpb24gb24gdGhlIG90aGVyIHNpZGUgb2YgdGhlIG1lbWJyYW5lIGlzwqA=aHlwZXJ0b25pYw==Lg==[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDt0aGUgc2FtZSBhbW91bnQgb2Ygc29sdXRlLiYjODIyMTsgV2hhdCBwcmVmaXggaXMgYXNzb2NpYXRlZCB3aXRoICYjODIyMDttb3JlJiM4MjIxOyBvciAmIzgyMjA7dG9vIG11Y2guJiM4MjIxOw==
Cg==[Qq]
[q]A solution that has a higher water concentration than a solution on the other side of the membrane is ___________
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiA=SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBMZXNzIHNvbHV0ZSBtZWFuc8KgaGlnaGVyIHdhdGVyIGNvbmNlbnRyYXRpb24u[Qq]
[f]Tm8uIEh5cGVydG9uaWMgbWVhbnMgaGlnaGVyIA==c29sdXRlIGNvbmNlbnRyYXRpb24uwqBJZiB0aGVyZSYjODIxNztzIG1vcmUgc29sdXRlLCB0aGVyZSBoYXMgdG8gYmUgbGVzcyB3YXRlci4gWW91JiM4MjE3O3JlIGxvb2tpbmcgZm9yIHRoZSB0ZXJtIHRoYXQgbWVhbnMgJiM4MjIwO2xvd2VyIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTs=[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSAob3Igd2F0ZXIpIGNvbmNlbnRyYXRpb24uJiM4MjIxO8KgSGlnaGVyIHdhdGVyIGNvbmNlbnRyYXRpb24gbWVhbnMgJiM4MjIwO2xvd2VyIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTsgV2hpY2ggdGVybSBtZWFuc8KgJiM4MjIwO2xvd2VyIHNvbHV0ZSBjb25jZW50cmF0aW9uPyYjODIyMTs=
Cg==[Qq]
[q]A solution that has the same solute concentration as a solution on the other side of the membrane is ___________
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG 9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3IgYSB0ZXJtwqB0aGF0IG1lYW5zICYjODIyMDt0aGUgc2FtZSBzb2x1dGXCoGNvbmNlbnRyYXRpb24uJiM4MjIxOyBXaGF0IHByZWZpeCBtZWFucyAmIzgyMjA7dGhlIHNhbWU/JiM4MjIxOyBUaGluayBvZiBhIHRyaWFuZ2xlIHRoYXQgaGFzIHR3byBzaWRlcyB0aGF0IGFyZSB0aGUgc2FtZSYjODIzMDs=[Qq]
[f]Tm8uIEh5cGVydG9uaWMgbWVhbnMgaGlnaGVyIA==c29sdXRlIGNvbmNlbnRyYXRpb24uwqBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3IgYSB0ZXJtwqB0aGF0IG1lYW5zICYjODIyMDt0aGUgc2FtZSBzb2x1dGXCoGNvbmNlbnRyYXRpb24uICYjODIyMDtXaGF0IHByZWZpeCBtZWFucyAmIzgyMjA7dGhlIHNhbWU/JiM4MjIxOyBUaGluayBvZiBhIHRyaWFuZ2xlIHRoYXQgaGFzIHR3byBzaWRlcyB0aGF0IGFyZSB0aGUgc2FtZSYjODIzMDs=[Qq]
[f]WWVzIcKgSXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTsgSnVzdCByZW1lbWJlciB0aGUgaXNvc2NlbGVzIHRyaWFuZ2xlLg==
Cg==[Qq]
[q]A solution that has a lower solute concentration than a solution on the other side of the membrane is ___________
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiA=SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7[Qq]
[f]Tm8uIA==SHlwZXJ0b25pYw==IG1lYW5zIA==aGlnaGVyIHNvbHV0ZSBjb25jZW50cmF0aW9uLiBUaGUgdGVybSB0aGF0IG1lYW5zIA==[Qq]lower solute concentration begins with a prefix that means “lower.”
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDt0aGUgc2FtZSBhbW91bnQgb2Ygc29sdXRlLiYjODIyMTsgVGhlIHRlcm0gdGhhdCBtZWFucyA=bG93ZXIgc29sdXRlIGNvbmNlbnRyYXRpb24=IGJlZ2lucyB3aXRoIGEgcHJlZml4IHRoYXQgbWVhbnMgJiM4MjIwO2xvd2VyLiYjODIyMTs=
[Qq][q]A solution that has a lower water concentration than a solution on the other side of the membrane is ___________
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBMZXNzIHNvbHV0ZSBtZWFuc8KgaGlnaGVyIHdhdGVyIGNvbmNlbnRyYXRpb24uIFdoYXQgdGVybSBtZWFucyAmIzgyMjA7aGlnaGVyIHNvbHV0ZSBjb25jZW50cmF0aW9uPyYjODIyMTs=[Qq]
[f]WWVzLiBIeXBlcnRvbmljIG1lYW5zIGhpZ2hlciA=c29sdXRlIGNvbmNlbnRyYXRpb24uwqBJZiB0aGVyZSYjODIxNztzIG1vcmUgc29sdXRlLCB0aGVyZSBoYXMgdG8gYmUgbGVzcyB3YXRlci4=[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSAob3Igd2F0ZXIpIGNvbmNlbnRyYXRpb24uJiM4MjIxOyBMb3dlcsKgd2F0ZXIgY29uY2VudHJhdGlvbiBtZWFucyAmIzgyMjA7aGlnaGVyIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTsgV2hpY2ggdGVybSBtZWFuc8KgJiM4MjIwO2hpZ2hlciBzb2x1dGUgY29uY2VudHJhdGlvbj8mIzgyMjE7
Cg==[Qq]
[q]A solution that has the same water concentration as a solution on the other side of the membrane is ___________
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG 9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3IgYSB0ZXJtwqB0aGF0IG1lYW5zICYjODIyMDt0aGUgc2FtZSBzb2x1dGXCoGNvbmNlbnRyYXRpb24mIzgyMjE7IChiZWNhdXNlIGlmIHRoZSBzb2x1dGUgY29uY2VudHJhdGlvbiBpcyB0aGUgc2FtZSwgdGhlIHdhdGVyIGNvbmNlbnRyYXRpb24gYWxzbyBoYXMgdG8gYmUgdGhlIHNhbWUpLiBXaGF0IHByZWZpeCBtZWFucyAmIzgyMjA7dGhlIHNhbWU/JiM4MjIxOyBUaGluayBvZiBhIHRyaWFuZ2xlIHRoYXQgaGFzIHR3byBzaWRlcyB0aGF0IGFyZSB0aGUgc2FtZSYjODIzMDs=[Qq]
[f]Tm8uIEh5cGVydG9uaWMgbWVhbnMgaGlnaGVyIA==c29sdXRlIGNvbmNlbnRyYXRpb24uwqBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3IgYSB0ZXJtwqB0aGF0IG1lYW5zICYjODIyMDt0aGUgc2FtZSBzb2x1dGXCoGNvbmNlbnRyYXRpb24mIzgyMjE7IChiZWNhdXNlIGlmIHRoZSBzb2x1dGUgY29uY2VudHJhdGlvbiBpcyB0aGUgc2FtZSwgdGhlIHdhdGVyIGNvbmNlbnRyYXRpb24gYWxzbyBoYXMgdG8gYmUgdGhlIHNhbWUpLiBXaGF0IHByZWZpeCBtZWFucyAmIzgyMjA7dGhlIHNhbWU/JiM4MjIxOyBUaGluayBvZiBhIHRyaWFuZ2xlIHRoYXQgaGFzIHR3byBzaWRlcyB0aGF0IGFyZSB0aGUgc2FtZSYjODIzMDs=[Qq]
[f]WWVzIcKgSXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSBjb25jZW50cmF0aW9uLiYjODIyMTvCoElmIHRoZSBzb2x1dGUgY29uY2VudHJhdGlvbiBpcyB0aGUgc2FtZSwgdGhlIHdhdGVyIGNvbmNlbnRyYXRpb24gd2lsbCBhbHNvIGJlIHRoZSBzYW1lLg==
Cg==[Qq]
[q]A gummy bear is placed in water. The gummy bear is made mostly of sugar, held together by gelatin. The gummy bear is ___________ to the water.
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7wqBZb3UmIzgyMTc7cmUgY29tcGFyaW5nIGEgZ3VtbXkgYmVhciwgd2l0aCBhbGwgb2YgaXRzIHN1Z2FyLCB0byBtb3N0bHkgcHVyZSB3YXRlci4gV2hhdCB3b3JkIG1lYW5zICYjODIyMDttb3JlIHNvbHV0ZT8mIzgyMjE7[Qq]
[f]RXhjZWxsZW50LsKgQSBndW1teSBiZWFyLCB3aXRoIGFsbCBvZiBpdHMgc3VnYXIswqBoYXMgYSBoaWdoZXIgc29sdXRlIGNvbmNlbnRyYXRpb24gdGhhbiB0aGUgd2F0ZXIgdGhhdCBpdCYjODIxNztzIGluLiDCoFRoYXQgbWFrZXMgdGhlIGd1bW15wqA=aHlwZXJ0b25pYw==IHRvIHRoZSB3YXRlci4=[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDt0aGUgc2FtZSBhbW91bnQgb2Ygc29sdXRlLiYjODIyMTsgWW91JiM4MjE3O3JlIGNvbXBhcmluZyBhIGd1bW15IGJlYXIsIHdpdGggYWxsIG9mIGl0cyBzdWdhciwgdG8gbW9zdGx5IHB1cmUgd2F0ZXIuIFdoYXQgd29yZCBtZWFucyAmIzgyMjA7bW9yZSBzb2x1dGU/JiM4MjIxOw==
Cg==[Qq]
[q]In this diagram, the cell is ________ to the solution outside the cell
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIA==SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7IExvb2sgYXQgaG93IG11Y2ggbW9yZSBzb2x1dGUgaXMgaW5zaWRlIHRoZSBjZWxsIHRoYW4gb3V0c2lkZS4gV2hhdCB0ZXJtIG1lYW5zICYjODIyMDtoaWdoZXIgc29sdXRlIGNvbmNlbnRyYXRpb24/JiM4MjIxOw==[Qq]
[f]WWVzLiBIeXBlcnRvbmljIG1lYW5zIGhpZ2hlciA=c29sdXRlIGNvbmNlbnRyYXRpb24uIEFzIHlvdSBjYW4gc2VlLCB0aGUgY2VsbCBpcyBoeXBlcnRvbmljIHRvIGl0cyBlbnZpcm9ubWVudC4=[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSAob3Igd2F0ZXIpIGNvbmNlbnRyYXRpb24uJiM4MjIxO8KgTG9vayBhdCBob3cgbXVjaCBtb3JlIHNvbHV0ZSBpcyBpbnNpZGUgdGhlIGNlbGwgdGhhbiBvdXRzaWRlLiBXaGF0IHRlcm0gbWVhbnMgJiM4MjIwO2hpZ2hlciBzb2x1dGUgY29uY2VudHJhdGlvbj8mIzgyMjE7
Cg==[Qq]
[q]In this diagram, the cell is ________ to the solution outside the cell
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiA=SHlwb3RvbmljIG1lYW5zICYjODIyMDtsZXNzIHNvbHV0ZS4mIzgyMjE7IEFzIHlvdSBjYW4gc2VlLCB0aGUgY2VsbCBpcyBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50Lg==[Qq]
[f]Tm8uIEh5cGVydG9uaWMgbWVhbnMgaGlnaGVyIA==c29sdXRlIGNvbmNlbnRyYXRpb24uIEp1c3QgYnkgbG9va2luZywgeW91IGNhbiB0ZWxsIHRoYXQgdGhlcmUmIzgyMTc7cyBhIGxvd2VyIGNvbmNlbnRyYXRpb24gb2Ygc29sdXRlIGluc2lkZSB0aGUgY2VsbCB0aGFuIG91dHNpZGUgdGhlIGNlbGwuIMKgV2hhdCB0ZXJtIG1lYW5zICYjODIyMDtsb3dlciBzb2x1dGUgY29uY2VudHJhdGlvbj8mIzgyMjE7[Qq]
[f]Tm8uIA==SXNvdG9uaWM=IG1lYW5zICYjODIyMDtzYW1lIHNvbHV0ZSAob3Igd2F0ZXIpIGNvbmNlbnRyYXRpb24uJiM4MjIxO8KgSnVzdCBieSBsb29raW5nLCB5b3UgY2FuIHRlbGwgdGhhdCB0aGVyZSYjODIxNztzIGEgbG93ZXIgY29uY2VudHJhdGlvbiBvZiBzb2x1dGUgaW5zaWRlIHRoZSBjZWxsIHRoYW4gb3V0c2lkZSB0aGUgY2VsbC4gwqBXaGF0IHRlcm0gbWVhbnMgJiM4MjIwO2xvd2VyIHNvbHV0ZSBjb25jZW50cmF0aW9uPyYjODIyMTs=
Cg==[Qq]
[q]In this situation, water will
[c]ZmxvdyBpbnRvIH RoZSBjZWxsLg==[Qq]
[c]ZmxvdyBvdXQgb2YgdGhlIGNlbGw=[Qq]
[c]ZmxvdyBpbnRvIGFuZCBvdXQgb2YgdGhlIGNlbGwgYXQgZXF1YWwgcmF0ZXMsIHdpdGggbm8gbmV0IGNoYW5nZS4=[Qq]
[f]WWVzLiBXYXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYy7CoEJlY2F1c2UgdGhlIGNlbGwgaXMgaHlwZXJ0b25pYyB0byBpdHMgZW52aXJvbm1lbnQsIHdhdGVyIHdpbGwgZmxvdyA=aW50bw==IHRoZSBjZWxsLg==[Qq]
[f]Tm8uIFdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLiBJcyB0aGlzIGNlbGwgaHlwZXJ0b25pYyBvciBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50Pw==[Qq]
[f]Tm8uIFdhdGVyIGZsb3cgd291bGQgYmUgZXF1YWwgaWYgdGhlIGNlbGwgd2VyZSBpc290b25pYyB0byBpdHMgZW52aXJvbm1lbnQsIGFuZCB0aGF0JiM4MjE3O3Mgbm90IHRoZSBjYXNlLiBLZWVwIGluIG1pbmQgdGhhdCB3YXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYywgYW5kIGZpZ3VyZSBvdXQgdGhlIGFuc3dlci4=
Cg==[Qq]
[q]In this situation, water will
[c]ZmxvdyBpbnRvIHRoZSBjZWxsLg==[Qq]
[c]ZmxvdyBvdXQgb2 YgdGhlIGNlbGw=[Qq]
[c]ZmxvdyBpbnRvIGFuZCBvdXQgb2YgdGhlIGNlbGwgYXQgZXF1YWwgcmF0ZXMsIHdpdGggbm8gbmV0IGNoYW5nZS4=[Qq]
[f]Tm8uIFdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLiBUaGUgaHlwb3RvbmljIHNpZGUgaXMgdGhlIG9uZSB3aXRoIGxlc3Mgc29sdXRlLiBOb3cgZmlndXJlIG91dCB0aGUgYW5zd2VyLg==[Qq]
[f]WWVzLiBXYXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYy4gQmVjYXVzZSB0aGUgY2VsbCBpcyBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50LCB3YXRlciB3aWxsIGZsb3cgb3V0IG9mwqB0aGUgY2VsbC4=[Qq]
[f]Tm8uIFdhdGVyIGZsb3cgd291bGQgYmUgZXF1YWwgaWYgdGhlIGNlbGwgd2VyZSBpc290b25pYyB0byBpdHMgZW52aXJvbm1lbnQsIGFuZCB0aGF0JiM4MjE3O3Mgbm90IHRoZSBjYXNlLiBLZWVwIGluIG1pbmQgdGhhdCB3YXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYywgYW5kIGZpZ3VyZSBvdXQgdGhlIGFuc3dlci4=
Cg==[Qq]
[q]Assume that this cell is an animal cell (without a cell wall). In this situation, the cell will
[c]aW5jcmVhc2Ug aW4gc2l6ZQ==[Qq]
[c]ZGVjcmVhc2UgaW4gc2l6ZQ==[Qq]
[c]cmVtYWluIHRoZSBzYW1lIHNpemUu[Qq]
[f]WWVzLiBXYXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYy7CoEFzIHdhdGVyIGZsb3dzIGludG8gdGhlIGh5cGVydG9uaWMgY2VsbCwgaXQgd2lsbCBpbmNyZWFzZSBpbiBzaXplLg==[Qq]
[f]Tm8uIFdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLiBJcyB0aGlzIGNlbGwgaHlwZXJ0b25pYyBvciBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50PyBGaWd1cmUgdGhhdCBvdXQsIHRoZW4gZmlndXJlIG91dCB0aGUgZGlyZWN0aW9uIG9mIHdhdGVyIGZsb3cu[Qq]
[f]Tm8uIFRoZSBjZWxsIHdvdWxkIHN0YXkgdGhlIHNhbWUgc2l6ZSBpZiBpdCB3ZXJlIGlzb3RvbmljIHRvIGl0cyBlbnZpcm9ubWVudC4gSXQmIzgyMTc7cyBub3QuIEtlZXAgaW4gbWluZCB0aGF0IHdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLCBhbmQgZmlndXJlIG91dCB0aGUgYW5zd2VyLg==
Cg==[Qq]
[q]Assume that this cell is an animal cell (without a cell wall). In this situation, the cell will
[c]aW5jcmVhc2UgaW4gc2l6ZQ==[Qq]
[c]ZGVjcmVhc2Ug aW4gc2l6ZQ==[Qq]
[c]cmVtYWluIHRoZSBzYW1lIHNpemUu[Qq]
[f]Tm8uIFdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLiBJcyB0aGlzIGNlbGwgaHlwZXJ0b25pYyBvciBoeXBvdG9uaWMgdG8gaXRzIGVudmlyb25tZW50PyBGaWd1cmUgdGhhdCBvdXQsIHRoZW4gZmlndXJlIG91dCB0aGUgZGlyZWN0aW9uIG9mIHdhdGVyIGZsb3cu[Qq]
[f]WWVzLiBXYXRlciBhbHdheXMgZmxvd3MgZnJvbSBoeXBvdG9uaWMgdG8gaHlwZXJ0b25pYy7CoEFzIHdhdGVyIGZsb3dzIG91dCBvZsKgdGhlIGh5cG90b25pY8KgY2VsbCwgaXQgd2lsbCBkZWNyZWFzZcKgaW4gc2l6ZS4=[Qq]
[f]Tm8uIFRoZSBjZWxsIHdvdWxkIHN0YXkgdGhlIHNhbWUgc2l6ZSBpZiBpdCB3ZXJlIGlzb3RvbmljIHRvIGl0cyBlbnZpcm9ubWVudC4gSXQmIzgyMTc7cyBub3QuIEtlZXAgaW4gbWluZCB0aGF0IHdhdGVyIGFsd2F5cyBmbG93cyBmcm9tIGh5cG90b25pYyB0byBoeXBlcnRvbmljLCBhbmQgZmlndXJlIG91dCB0aGUgYW5zd2VyLg==
Cg==[Qq]
[q]When plant cells are in this environment, they’ll be the most solid and firm.
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiBXaGVuIHBsYW50IGNlbGxzIGFyZSBpbiBhIGh5cG90b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgZmxvd3MgaW50byB0aGUgY2VsbHMuIFRoaXMgZXhwYW5kcyB0aGUgY2VudHJhbCB2YWN1b2xlIGFuZCBwdXNoZXMgdGhlIG1lbWJyYW5lIGFnYWluc3QgdGhlIHdhbGwuIFRoYXQgbWFrZXMgdGhlIGNlbGxzIChhbmQgdGhlIGVudGlyZSBwbGFudCkgc29saWQgYW5kIGZpcm0u
Cg==[Qq]
[f]Tm8uIFdoZW4gcGxhbnQgY2VsbHMgYXJlIGluIGEgaHlwZXJ0b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgZmxvd3Mgb3V0IG9mIHRoZSBjZWxscy4gVGhpcyBjb250cmFjdHMgdGhlIGNlbnRyYWwgdmFjdW9sZSBhbmQgcGVlbHMgdGhlIG1lbWJyYW5lIGF3YXkgZnJvbSB0aGUgd2FsbC4gVGhhdCBtYWtlcyB0aGUgY2VsbHMgKGFuZCB0aGUgZW50aXJlIHBsYW50KSBkcm9vcHkgYW5kIHdpbHRlZC4=
Cg==[Qq]
[f]Tm8gKGJ1dCB0aGlzIGlzIHRoZSBzZWNvbmQtYmVzdCBhbnN3ZXIpLiBJZiBwbGFudCBjZWxscyBhcmUgaW4gYW4gaXNvdG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIHdpbGwgYmUgZW50ZXJpbmcgYW5kIGxlYXZpbmcgdGhlbSBhdCB0aGUgc2FtZSByYXRlLiBUaGUgY2VsbHMgd2lsbCBiZSBpbiByZWFzb25hYmx5IGdvb2Qgc2hhcGUsIGJ1dCBhIGNoYW5nZSBpbiB0aGVpciBvc21vdGljIGNvbmRpdGlvbiB3b3VsZCBtYWtlIHRoZSBwbGFudCBldmVuIG1vcmUgc29saWQgYW5kIGZpcm0u
Cg==Cg==[Qq]
[q]When animal cells are in this environment, they’ll shrink and shrivel
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIFdoZW4gYW5pbWFsIGNlbGxzIGFyZSBpbiBhIGh5cG90b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgZmxvd3MgaW50byB0aGUgY2VsbHMuIFN0dWR5IHRoZSBkaWFncmFtIGJlbG93IHRvIHNlZSB3aGF0IGNvbmRpdGlvbnMgd2lsbCBjYXVzZSBhbmltYWwgY2VsbHMgdG8gc2hyaW5rIGFuZCBzaHJpdmVsLg==
Cg==[Qq]
[f]WWVzLiBXaGVuIGFuaW1hbCBjZWxscyBhcmUgaW4gYSBoeXBlcnRvbmljIGVudmlyb25tZW50LCB3YXRlciBmbG93cyBvdXQgb2YgdGhlIGNlbGxzLiBUaGlzIG1ha2VzIHRoZW0gc2hyaW5rIGFuZCBzaHJpdmVsLg==
Cg==[Qq]
[f]Tm8uIElmIGFuaW1hbMKgY2VsbHMgYXJlIGluIGFuIGlzb3RvbmljIGVudmlyb25tZW50LCB3YXRlciB3aWxsIGJlIGVudGVyaW5nIGFuZCBsZWF2aW5nIHRoZW0gYXQgdGhlIHNhbWUgcmF0ZS7CoFN0dWR5IHRoZSBkaWFncmFtIGJlbG93IHRvIHNlZSB3aGF0IGNvbmRpdGlvbnMgd2lsbCBjYXVzZSBhbmltYWwgY2VsbHMgdG8gc2hyaW5rIGFuZCBzaHJpdmVsLg==
Cg==Cg==[Qq]
[q]When a plant is in this environment, it wilts.
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0 b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]Tm8uIFdoZW4gcGxhbnQgY2VsbHMgYXJlIGluIGEgaHlwb3RvbmljIGVudmlyb25tZW50LCB3YXRlciBmbG93cyBpbnRvIHRoZSBjZWxscy4gVGhpcyBleHBhbmRzIHRoZSBjZW50cmFsIHZhY3VvbGUgYW5kIHB1c2hlcyB0aGUgbWVtYnJhbmUgYWdhaW5zdCB0aGUgd2FsbC4gVGhhdCBtYWtlcyB0aGUgY2VsbHMgKGFuZCB0aGUgZW50aXJlIHBsYW50KSBzb2xpZCBhbmQgZmlybS4=
Cg==[Qq]
[f]WWVzLiBXaGVuIHBsYW50IGNlbGxzIGFyZSBpbiBhIGh5cGVydG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIGZsb3dzIG91dCBvZiB0aGUgY2VsbHMuIFRoaXMgY29udHJhY3RzIHRoZSBjZW50cmFsIHZhY3VvbGUgYW5kIHBlZWxzIHRoZSBtZW1icmFuZSBhd2F5IGZyb20gdGhlIHdhbGwuIFRoYXQgbWFrZXMgdGhlIGNlbGxzIChhbmQgdGhlIGVudGlyZSBwbGFudCkgZHJvb3B5IGFuZCB3aWx0ZWQu
Cg==[Qq]
[f]Tm8uIElmIHBsYW50IGNlbGxzIGFyZSBpbiBhbiBpc290b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgd2lsbCBiZSBlbnRlcmluZyBhbmQgbGVhdmluZyB0aGVtIGF0IHRoZSBzYW1lIHJhdGUuwqBUaGUgY2VsbHMgd2lsbCBiZSBpbiByZWFzb25hYmx5IGdvb2Qgc2hhcGUuIFdoYXQgb3Ntb3RpYyBjb25kaXRpb24gd291bGQgY2F1c2Ugd2F0ZXIgdG8gbGVhdmUgdGhlIGNlbGxzLCBjYXVzaW5nIHRoZSBwbGFudCB0byB3aWx0Pw==
Cg==Cg==[Qq]
[q]When animal cells are in this environment, they’ll expand, then burst.
[c]aHlwb3 Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG9uaWM=[Qq]
[f]WWVzLiBXaGVuIGFuaW1hbCBjZWxscyBhcmUgaW4gYSBoeXBvdG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIGZsb3dzIGludG8gdGhlIGNlbGxzLsKgVGhpcyBjYXVzZXMgdGhlbSB0byBleHBhbmQsIGFuZCBldmVudHVhbGx5IGJ1cnN0Lg==
Cg==[Qq]
[f]Tm8uIFdoZW4gYW5pbWFsIGNlbGxzIGFyZSBpbiBhIGh5cGVydG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIGZsb3dzIG91dCBvZiB0aGUgY2VsbHMuIFRoaXMgbWFrZXMgdGhlbSBzaHJpbmsgYW5kIHNocml2ZWwuIFdoYXQgb3Ntb3RpYyBjb25kaXRpb24gY2F1c2VzIHRoZW0gdG8gZXhwYW5kIGFuZCBidXJzdD8=
Cg==[Qq]
[f]Tm8uIElmIGFuaW1hbMKgY2VsbHMgYXJlIGluIGFuIGlzb3RvbmljIGVudmlyb25tZW50LCB3YXRlciB3aWxsIGJlIGVudGVyaW5nIGFuZCBsZWF2aW5nIHRoZW0gYXQgdGhlIHNhbWUgcmF0ZS4gVGhlaXIgc2l6ZSByZW1haW5zIHRoZSBzYW1lLiBTdHVkeSB0aGUgZGlhZ3JhbSBiZWxvdyB0byBzZWUgd2hhdCBjb25kaXRpb25zIHdpbGwgY2F1c2UgYW5pbWFsIGNlbGxzIHRvIGV4cGFuZCBhbmQgYnVyc3Q/
Cg==Cg==[Qq]
[q]If you want to keep animal tissue or cells alive outside the body, you have to keep them in what kind of osmotic environment?
[c]aHlwb3Rvbmlj[Qq]
[c]aHlwZXJ0b25pYw==[Qq]
[c]aXNvdG 9uaWM=[Qq]
[f]Tm8uIFdoZW4gYW5pbWFsIGNlbGxzIGFyZSBpbiBhIGh5cG90b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgZmxvd3MgaW50byB0aGUgY2VsbHMuwqBUaGlzIGNhdXNlcyB0aGVtIHRvIGV4cGFuZCwgYW5kIGV2ZW50dWFsbHkgYnVyc3QuIFdoYXQgY29uZGl0aW9uIGtlZXBzIHRoZW0gdGhlIHNhbWUgc2l6ZSAod2hpY2ggaXMgdGhlIGhlYWx0aGllc3QgY29uZGl0aW9uIGZvciB0aGUgY2VsbHMpPw==
Cg==[Qq]
[f]Tm8uIFdoZW4gYW5pbWFsIGNlbGxzIGFyZSBpbiBhIGh5cGVydG9uaWMgZW52aXJvbm1lbnQsIHdhdGVyIGZsb3dzIG91dCBvZiB0aGUgY2VsbHMuIFRoaXMgbWFrZXMgdGhlbSBzaHJpbmsgYW5kIHNocml2ZWwuIFdoYXQgY29uZGl0aW9uIGtlZXBzIHRoZW0gdGhlIHNhbWUgc2l6ZSAod2hpY2ggaXMgdGhlIGhlYWx0aGllc3QgY29uZGl0aW9uIGZvciB0aGUgY2VsbHMpPw==
Cg==[Qq]
[f]WWVzLiBJZiBhbmltYWzCoGNlbGxzIGFyZSBpbiBhbiBpc290b25pYyBlbnZpcm9ubWVudCwgd2F0ZXIgd2lsbCBiZSBlbnRlcmluZyBhbmQgbGVhdmluZyB0aGVtIGF0IHRoZSBzYW1lIHJhdGUuIFRoZWlyIHNpemUgcmVtYWlucyB0aGUgc2FtZSwgd2hpY2ggaXMgdGhlIGhlYWx0aGllc3QgY29uZGl0aW9uIGZvciB0aGUgY2VsbHMu
Cg==Cg==[Qq]
[x]
[restart]
[/qwiz]
Next
This (at least for now) ends this series of tutorials about cell membranes and osmosis. Here are some possible next moves.
- To extend and deepen your understanding of water flow in living organisms, use my tutorial on Water Transport in Plants. That tutorial also introduces the concept of water potential, an idea that’s part of the AP Biology curriculum (Topic 2.8) and which is interesting in its own right).
- Proceed to Enzymes (the next module in my biology course)
- Use the menu above to choose another module.
- Watch Osmosis! (Music Video) or buy Osmosis! on iTunes or Amazon.
