1. Introduction: The Brave New World of Human Genetic Modification is Here
On November 25, 2018, Dr. He Jiankui, a scientist working in China, announced that he had created the first genetically edited human babies.
Dr. He used CRISPR-Cas9, the technology we’ll be discussing in this tutorial, to disable a gene called CCR5 in two twin girls, increasing their resistance to infection by HIV.
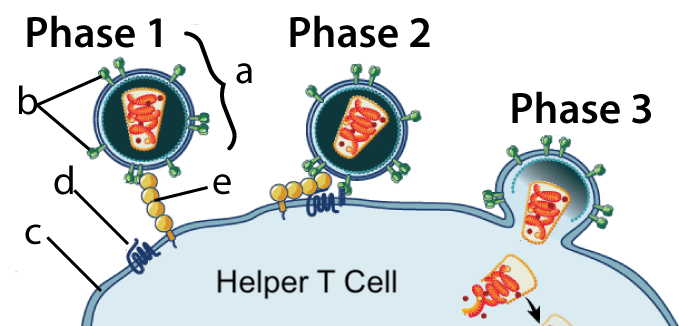
The CCR5 gene codes for a receptor protein (also called CCR5) that HIV (the virus that causes AIDS) uses to infect cells.
In the diagram at left, Phase 1 shows HIV bumping into a Helper T Cell. The Helper T cell is like the general in our immune system’s army. HIV has a surface protein, gp120, shown at “b,” that binds with a protein called CD4 (letter “e”) on the Helper T Cell’s membrane (c).
In Phase 2, the bound CD4 protein forms a complex with the CCR5 protein (at “d”). The formation of this complex induces receptor-mediated endocytosis (shown in Phase 3). In subsequent phases (not shown) HIV integrates itself into the Helper T cell’s nucleus, transforming the cell into an HIV factory. Ultimately, this kills the T-cell and destroys an infected person’s immune system. (If you want to learn more, click here for my tutorials about the immune system, and here for a tutorial about the life cycle of H.I.V. All links on this page will open in a separate tab.)
Many humans (including about 10% of Europe’s population) have a natural resistance to HIV infection because they carry a mutation in the CCR5 gene. That mutation results in a much smaller version of the CCR5 protein that doesn’t sit on the outside of the membrane, blocking the entry of HIV into helper T cells (use this link to read about CCR5 on Wikipedia).
The twins were born to a Chinese couple in which the father was HIV positive. Dr. He explains in this youtube video that his motive was to protect the girls from HIV infection and subsequent discrimination and that the birth of these girls had given the twins’ father “a reason to live.”
The response to Dr. He’s work was worldwide condemnation (see this report from the Associated Press, this article in the NY Times, and this article on Wikipedia. While the Chinese press initially lauded Dr. He’s accomplishments, he was subsequently investigated for ethical misconduct by the Chinese government, fired from a professorship at his university, fined, and imprisoned.
Why? Human gene editing of any type opens up many ethical issues, and Dr. He had taken it upon himself to cross a line that humanity hasn’t come to any clear agreement about. Additionally, the type of edit that Dr. He carried out was the one that people worry most about. It wasn’t merely a change in the cells of body tissues to cure a disease (a proposed therapy that I’ll describe below). Rather, Dr. He had changed the genome of every cell in the body of these twins, including their germ cells. If these girls reproduce, they can pass on the edited gene to their offspring. That kind of change brings up questions for which there are no clear answers:
- What are the conditions (if any) that justify human genetic modification? For example, is it okay to do gene-editing to prevent a couple from passing on a genetic disease like hemophilia or cystic fibrosis to their children? Is it okay for a couple to ensure that their child will have curly hair? Brown eyes?
- What are the risks that genetic editing brings to the offspring?
- What will the socio-economic consequences of genetic editing be? Are we paving a path to a world where there will be those who can afford to genetically modify their children and those who can’t? Are there ways to ensure that the benefits of this technology are used wisely, and distributed in a way that makes our world more just and equitable?
Those are some of the big issues. But even on a much more superficial and technical level, Dr. He’s work was medically unjustifiable. First, the girls were not at risk for HIV infection, and editing their genomes to prevent the expression of the CCR5 gene was unnecessary. Many well-established measures could have been used to protect them from HIV infection in utero and during birth. Second, genetic modification entails unknown risks: other genes in the girls’ genomes might have been affected (though Dr. He has made assurances that this is not the case). Third, in one of the twins, the gene edit was only partially successful: one allele still contains the working CCR5 gene, meaning that she’ll express the protein, and be just as much at risk of contracting HIV as an adult as anyone else.
With CRISPR-Cas9, modifying the genome of any organism — humans included — has become easier than ever before. To get an overview of how CRISPR works, watch the video below and then answer the questions that follow.
2. Overview Video of CRISPR-Cas9
3. CRISPR-Cas9 Video Quiz
[qwiz qrecord_id=”sciencemusicvideosMeister1961-CRISPR 1 (Video) M17″]
[h]Genome editing with CRISPR-Cas9: Video Quiz
[i]
[q] CRISPR first evolved as a system that bacteria used to protect themselves from attack from [hangman].
[c]IHZpcnVzZXM=[Qq]
[q] When CRISPR-containing bacteria are attacked by a virus, they produce two types of [hangman]. These molecules form a complex with a protein that’s called [hangman].
[c]IFJOQQ==[Qq]
[c]IGNhczk=[Qq]
[q multiple_choice=”true”] Cas9 is a nuclease. That means that it’s an enzyme that’s capable of cutting up
[c]IHBob3NwaG9saXBpZHM=[Qq]
[f]IE5vLiBUaGluayBhYm91dCB3aGF0IHZpcnVzZXMgaW5qZWN0IGludG8gY2VsbHMgd2hlbiB0aGV5IGF0dGFjayB0aGVtLg==[Qq]
[c]IHByb3RlaW5z[Qq]
[f]IE5vLiBUaGluayBhYm91dCB3aGF0IHZpcnVzZXMgaW5qZWN0IGludG8gY2VsbHMgd2hlbiB0aGV5IGF0dGFjayB0aGVtLg==[Qq]
[c]IA ==bnVjbGVpYyBhY2lkcw==[Qq]
[f]RXhjZWxsZW50ISBDYXM5IGlzIGEgbnVjbGVhc2UsIGFuIGVuenltZSBjYXBhYmxlIG9mIGN1dHRpbmcgdXAgRE5BLg==[Qq]
[q] CRISPR-Cas9 uses guide [hangman] to find matching DNA from a [hangman]. When it does, it makes a double-stranded cut in the viral [hangman], which disables the virus and keeps it from destroying the cell.
[c]IFJOQQ==[Qq]
[c]IHZpcnVz[Qq]
[c]RE5B[Qq]
[q]In recent years, scientists have engineered the CRISPR-Cas9 system so that instead of only cutting DNA from a [hangman], it can cut any targeted sequence of [hangman] in any organism. All that was required was changing the [hangman] RNA.
[c]dmlydXM=[Qq]
[c]RE5B[Qq]
[c]Z3VpZGU=[Qq]
[q multiple_choice=”true”] After the Cas9 enzyme cuts up targeted DNA in a cell, the cell
[c]IGluaXRpYXRlcyBjZWxsIHN1aWNpZGUgcGF0aHdheXMgKGFwb3B0b3NpcykgdGhhdCByZXN1bHQgaW4gbHlzb3NvbWFsIHJ1cHR1cmUgYW5kIGV2ZW50dWFsIGNlbGwgZGVhdGgu[Qq]
[f]IE5vLiBBcG9wdG9zaXMgaXMgYSByZWFsIHBoZW5vbWVub24sIGJ1dCBjZWxscyB1c3VhbGx5IHNhdmUgdGhhdCBtb3ZlIGFzIGEgbGFzdCByZXNvcnQuIE5leHQgdGltZSwgY2hvb3NlIGFub3RoZXIgYW5zd2VyLg==[Qq]
[c]IHJlY29ubmVjdHMgdGhlIGJyb2tlbiBETkEsIGJ1dCBvZnRlbiBpbiBhIHdheSB0 aGF0IHJlc3VsdHMgaW4gbXV0YXRpb25zIHRoYXQgZGlzYWJsZSB0aGUgZ2VuZS4=[Qq]
[f]IFRoYXQmIzgyMTc7cyBleGFjdGx5IHJpZ2h0LiBDZWxscyByZXNwb25kIHRvIEROQSBicmVha3MgdGhyb3VnaCBETkEgcmVwYWlyIG1lY2hhbmlzbXMuIE9uZSBtZWNoYW5pc20gcmVjb25uZWN0cyB0aGUgRE5BLCBidXQgb2Z0ZW4gaW4gYSB3YXkgdGhhdCBkaXNhYmxlcyB0aGUgZ2VuZS4=[Qq]
[c]IHN0YXJ0cyBkaXZpZGluZyBvdXQgb2YgY29udHJvbCwgb2Z0ZW4gYmVjb21pbmcgYSB0dW1vciB0aGF0IGNvdWxkIGRldmVsb3AgaW50byBjYW5jZXIu[Qq]
[f]Tm8uIFRoZXJlIGlzIGEgY29ubmVjdGlvbiBiZXR3ZWVuIG11dGF0aW9uIGFuZCBjYW5jZXIsIGJ1dCB0aGVyZSYjODIxNztzIGEgYmV0dGVyIGFuc3dlci4=[Qq]
[q]If scientists add working DNA to a cell where CRISPR-Cas9 is at work, the cell might cut out a defective gene and [hangman] in a working copy of that gene (hint: think of how you cut and ______ words in a word processor).
[c]cGFzdGU=[Qq]
[q]If CRISPR-Cas9 and foreign [hangman] are introduced to a fertilized egg, the result can be a transgenic organism.
[c]RE5B[Qq]
[/qwiz]
4. Understanding CRISPR
As you just saw, CRISPR-Cas9 evolved as a molecular defense used by bacteria to fight off attacks from bacteriophages (viruses that attack bacteria).
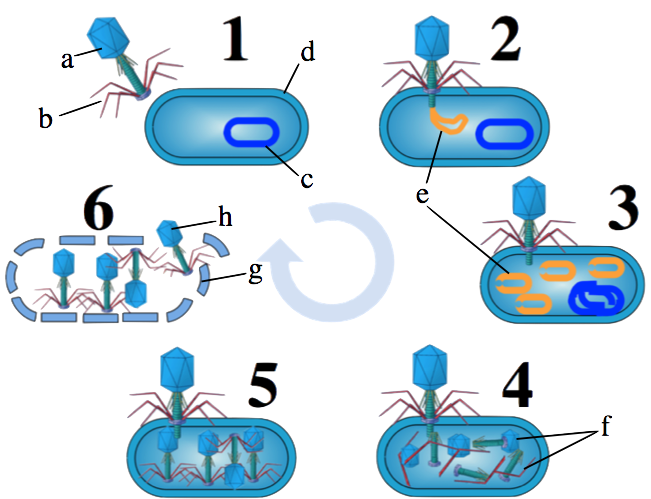
Viruses are constantly attacking bacteria. That’s one part of an unending war that has been going on between bacteria and viruses for billions of years. The viral attack plan is captured in the diagram at left, which shows a process known as the lytic cycle. Note that if you want to know more about viruses, you can do so at this tutorial.
When a virus (“a” and “b”) attacks a bacterial cell (d), the virus injects its DNA (e) into its bacterial victim. The viral DNA converts the bacterial cell into a virus factory (steps 3 and 4). This process begins as the virus shuts down the bacteria’s chromosome (at “c”). Next, the virus uses the bacterial cell’s molecular machinery (such as polymerases and ribosomes) to assemble new viruses (step 5). At some point, the bacterial cell becomes so full of viruses that the newly assembled viruses (h) burst out of the cell (step 6). This last step is called lysis, from which the lytic cycle gets its name.
Bacteria have survived this onslaught because of various countermeasures. These include restriction enzymes and the CRISPR-Cas9 system. Both systems are capable of detecting injected viral DNA and destroying it by breaking it apart. The CRISPR-Cas9 system, however, doesn’t only destroy viral DNA: it’s also a part of a system that remembers viral invaders, making the bacterium (and its descendants) more efficient at fending off future attacks from the same virus. CRISPR-Cas9, in other words, is like our immune system. It remembers previous attacks and becomes more efficient at fighting off future ones.
CRISPR is an acronym that stands for clustered regularly interspersed short palindromic repeats. Along a bacterial chromosome (“c,” above, “A” below) are clusters of repeated sequences (B), interspersed with what are called “spacers” (C). The spacers are non-repetitive DNA, and they consist of fragments of invading viral DNA.
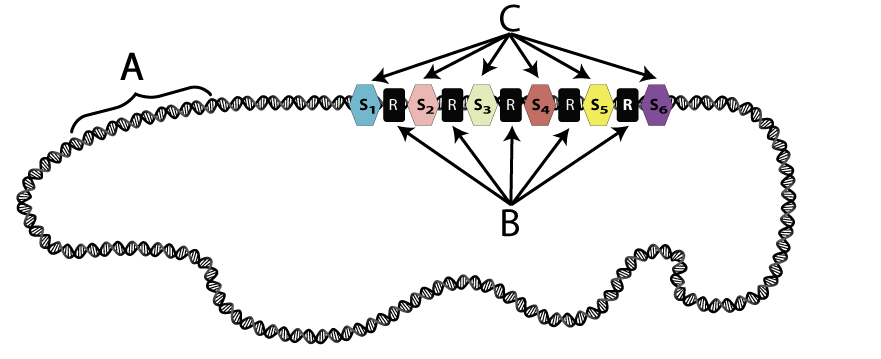
How does this viral DNA become integrated into a bacterial chromosome?
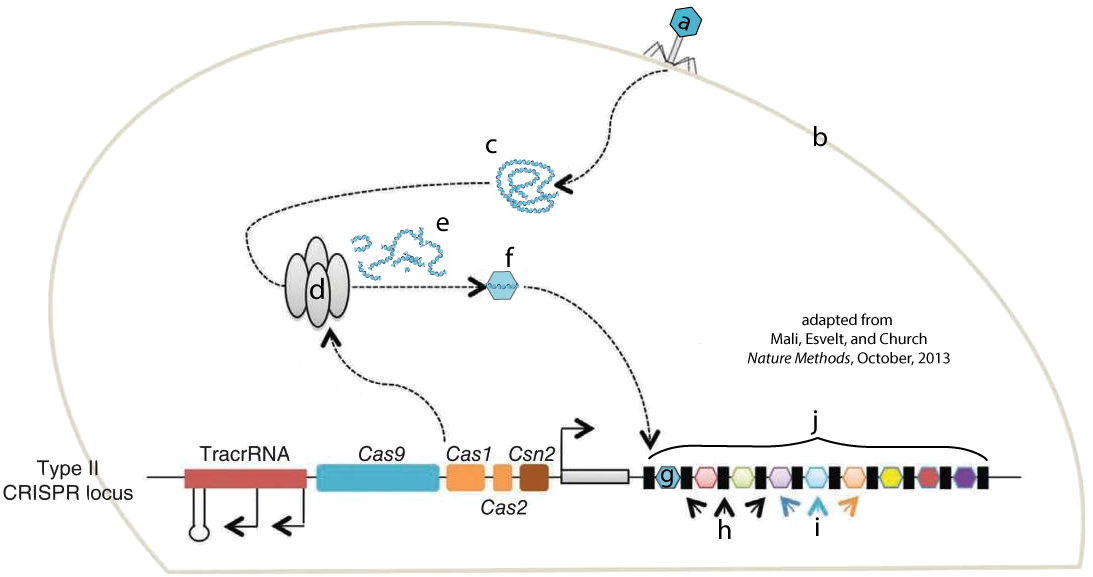
The image at left shows a bacteriophage (a) injecting its DNA (c) into a bacterial cell (b). Nuclease enzymes that are part of a CRISPR-associated complex (also known as Cas Complex enzymes, shown at “d”) attack the viral DNA, breaking it into fragments (e). One of these fragments (f) becomes incorporated into the CRISPR array (j) as a new spacer (g). The letter “i” shows spacers that were incorporated into the CRISPR array after previous infections. “H” shows repeats.
The Cas nuclease complex shown above at “d” is a kind of innate, or non-specific, immunity. It’s part of a bacterium’s generalized arsenal for fighting off bacteriophages. Once the new spacer (g) has been integrated into the CRISPR array, however, the cell has a kind of acquired immunity against further attack from this virus. Here’s how this works. Note that you have to scroll the text below the diagram to read the entire explanation.
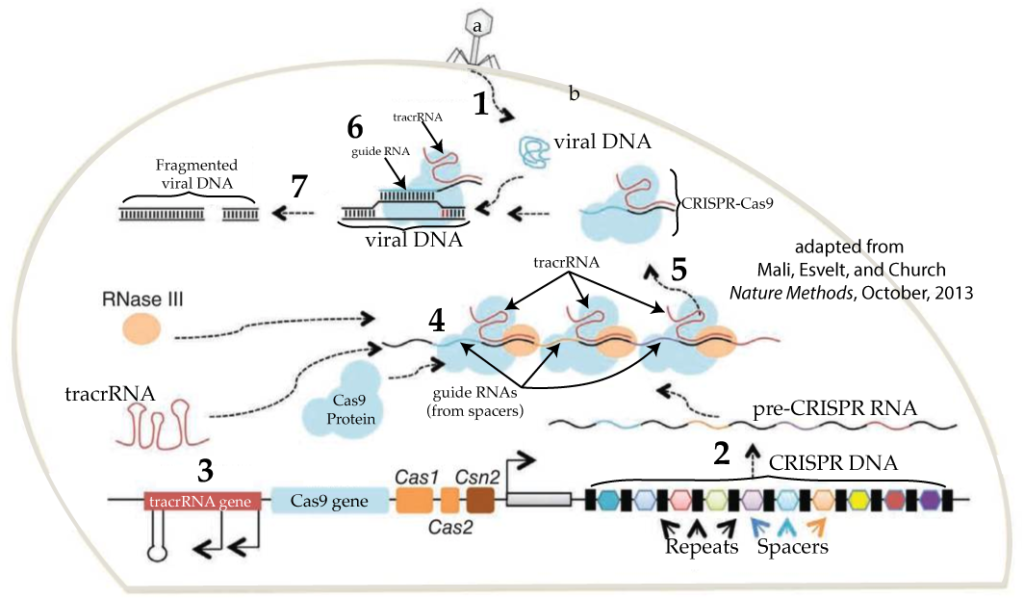
- STEP 1: A phage (at “a”) attacks the cell by injecting its DNA. Note that an identical phage had previously attacked this cell (or one of its ancestors), and a spacer with a fragment of this phage’s DNA is in the cell’s CRISPR DNA (at bottom right).
- STEP 2: Because a clone of this phage had previously attached the cell, the cell now has a new weapon with which to counterattack. The cell transcribes its CRISPR DNA into pre-CRISPR RNA. Note that CRISPR is an operon: a series of genes that get transcribed together into a single RNA transcript, labeled in this diagram as “pre-CRISPR RNA.”
- STEP 3: The cell’s counterattack requires transcription of additional genes. One of these is the Cas9 gene (shown a little bit to the right of and below number 3). As we learned above, Cas9 is a nuclease. With proper guidance from CRISPR RNA, it will be able to attack and destroy the viral DNA that’s been injected into the cell. An additional gene is the tracrRNA gene, which is transcribed into tracrRNA. As you can see below, tracrRNA bonds with mature CRISPR RNA to form a functional CRISPR-Cas9 complex. In some sources, it’s described as a “handle” that Cas9 holds on to as it carries out viral target surveillance and destruction. Study the image below to see all of the components of CRISPR-Cas9.
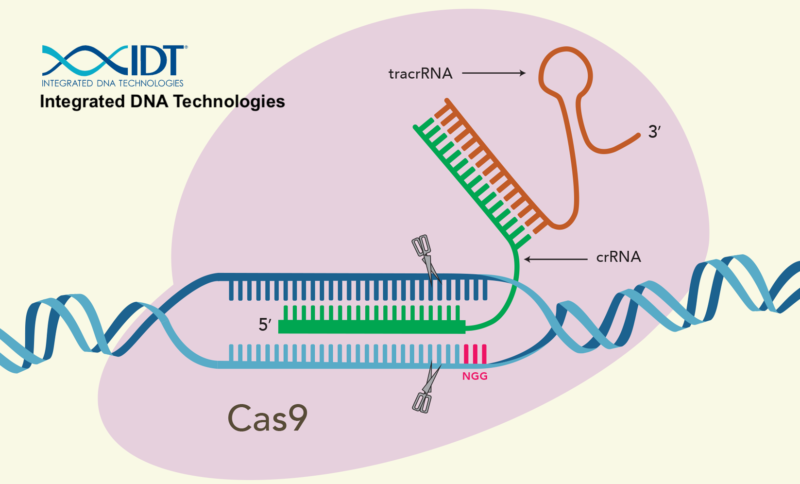
- STEP 4: Multiple CRISPR-Cas9 complexes form as the Cas9 protein, the RNA transcribed from the spacer DNA, and the tracrRNA all come together. Once associated with Cas9, the spacer RNA now graduates to become crRNA. The crRNA is the guide that the complex uses to find injected phage DNA. RNAase III is yet another enzyme that edits the various RNAs into their mature forms.
- STEP 5: Of the various CRISPR-Cas9 complexes built, one has the crRNA that complements a 20 nucleotide sequence in the DNA from the invading phage. That stretch of recognizable DNA is called a protospacer.
- STEP 6: When the CRISPR-Cas9 encounters phage DNA, the viral DNA is split open and lined up against the crRNA. If the crRNA complements the protospacer in the viral DNA …
- STEP 7: … then the viral DNA is broken apart, preventing the virus from taking over the cell.
5. Understanding CRISPR-Cas9: Interactive Diagrams
[qwiz style=”width: 700px !important;” qrecord_id=”sciencemusicvideosMeister1961-CRISPR 2, Interactive Diagrams (M17)”]
[q labels = “top”]
[l]Cas Nucleases
[fx] No. Please try again.
[f*] Excellent!
[l]cell wall/membrane
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]Fragmented Viral DNA
[fx] No. Please try again.
[f*] Correct!
[l]Insertion of New Spacer
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]Newest spacer
[fx] No. Please try again.
[f*] Correct!
[l]Older spacers
[fx] No. Please try again.
[f*] Correct!
[l]phage
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]Repeats
[fx] No. Please try again.
[f*] Great!
[l]viral DNA
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]
[l]Cas9
[fx] No. Please try again.
[f*] Great!
[l]Cas9 genes
[fx] No. Please try again.
[f*] Good!
[l]Cell membrane/wall
[fx] No. Please try again.
[f*] Excellent!
[l]CRISPR DNA
[fx] No, that’s not correct. Please try again.
[f*] Excellent!
[l]crRNA
[fx] No. Please try again.
[f*] Great!
[l]Fragmented Viral DNA
[fx] No. Please try again.
[f*] Good!
[l]Mature CRISPR-Cas9
[fx] No. Please try again.
[f*] Excellent!
[l]pre-CRISPR RNA
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]Phage
[fx] No. Please try again.
[f*] Correct!
[l]repeats
[fx] No. Please try again.
[f*] Great!
[l]spacers
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]tracrRNA
[fx] No. Please try again.
[f*] Correct!
[l]tracrRNA genes
[fx] No. Please try again.
[f*] Correct!
[l]Viral DNA
[fx] No. Please try again.
[f*] Good!
[q labels = “top”]
[l]Cas9
[fx] No. Please try again.
[f*] Good!
[l]crRNA
[fx] No. Please try again.
[f*] Great!
[l]tracrRNA
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]viral DNA
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[/qwiz]
6. CRISPR-Cas9 Becomes a Genome Editing System
By 2012, the mechanisms involved in the CRISPR-Cas9 system were well understood. This included understanding how CRISPR-Cas9 can target viral DNA (the protospacer), but not target the spacer DNA derived from protospacer/viral DNA. The key to this involves a short stretch of DNA known as the PAM, for Protospacer Adjacent Motif. The PAM is a 2-6 base pair DNA sequence, such as 5′-NGG-3′ (two guanine nucleotides, preceded by any nucleotide base (N) at the 5′ end). In CRISPR-Cas9 systems found in Staphylococcus pyogenes (a bacterial species in which CRISPR systems were widely studied), Cas9 will only cut viral DNA that contains this sequence. The genomic trick that’s carried out by bacterial systems with CRISPR-based immunity is that the spacer sequence does not contain the PAM, protecting the bacterial cell’s own DNA from its Cas9 enzymes. (various sources, including Wikipedia and this youtube video.)
So if we look again at CRISPR-Cas9, we can now understand all of its parts.
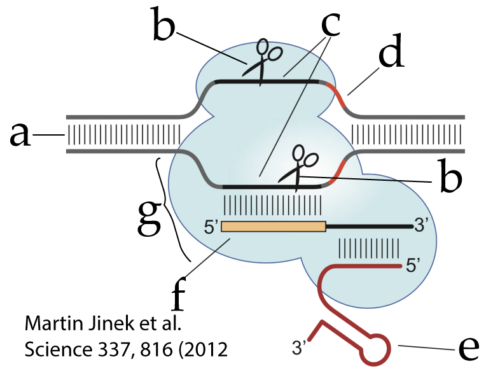
- “g” is the Cas9 protein
- “e” is the tracrRNA (the handle)
- “f” is crRNA, which includes the spacer RNA that recognizes the protospacer sequence (c) in the invading viral DNA (a)
- “d” is the PAM sequence, the molecular signal that tells Cas9 that this is viral RNA and should be cut
- “b” (which appears twice) represents the nucleases that make a double-stranded cut in the viral DNA.
With the science understood, a flash of insight followed. If CRISPR RNA enables the CRISPR-Cas9 system to recognize viral DNA and make a double-stranded cut, then why not re-engineer crRNA to target any DNA sequence? This insight emerged in several labs and is credited to various scientists, including Jennifer Doudna, Emmanuelle Charpentier, Feng Zhang, and George Church.
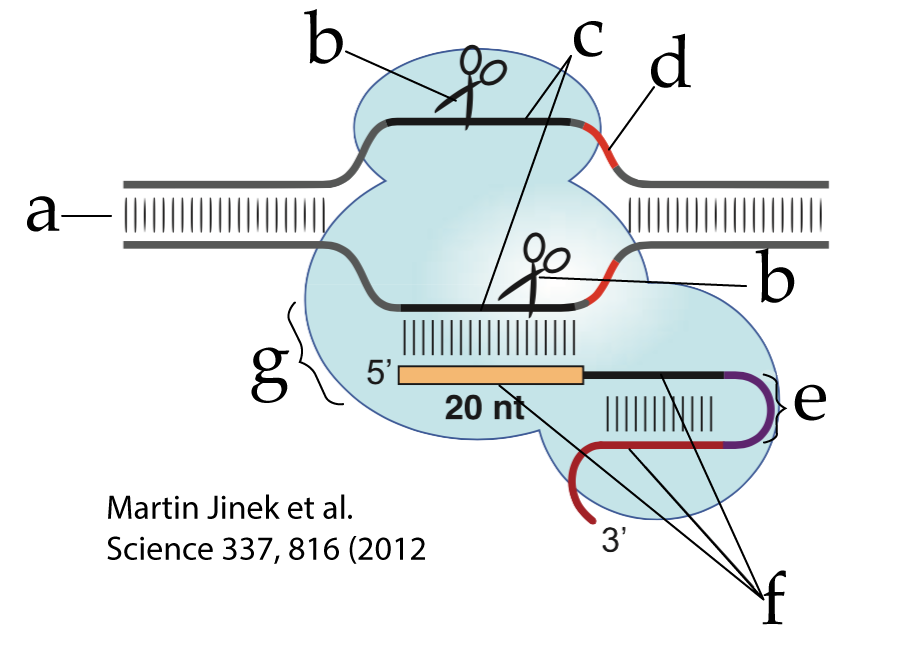
The CRISPR-Cas9 used in genetic engineering has been simplified from its form in nature by combining the tracrRNA with the crRNA into a single strand that’s called single guide RNA (sgRNA). This sgRNA is shown at “f”. The RNA segment that joins what previously had been the tracrRNA (at the 3′ end of the sgRNA) and the crRNA (at the 5′ end) is called a linker loop (at “e”). Everything else is the same (though note that some of the lettering in this diagram is different from that in the previous figure).
CRISPR-Cas9’s power relates to how easy it makes editing the genes of any organism. Before CRISPR-Cas9, other gene editing systems involved designing proteins that would find specific DNA sequences. Engineering these DNA-binding proteins was both expensive and time-consuming. By contrast, designing custom-made guide RNA for CRISPR is comparatively quick and easy (you can read this blog post from the Jackson Lab for a comparison of CRISPR with some of the previous techniques).
Paired with sequencing technology (click here to learn how sequencing is done), researchers can identify the sequence for any known gene, and target that gene by creating a custom sgRNA that seeks out the target sequence and makes a double-stranded cut.
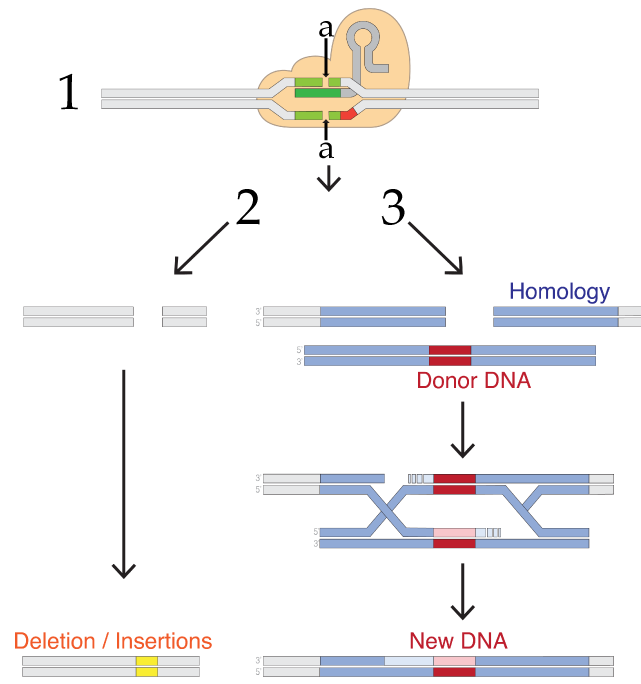
What happens next depends on the researcher’s goals. What follows lists only a few options, but keep in mind that the technology is changing rapidly.
If like Dr. He, the goal is to disable a gene, then you allow what’s shown as process number 2 in the diagram to the right. In any cell, a team of enzymes monitors the DNA for double-stranded breaks, and then works to recombine the broken DNA by reconnecting the broken strands. The type of repair shown on the left is called non-homologous end joining, a term that only makes sense if you compare it to the system on the right side (number 3).
Any sexually reproducing organism inherits one allele from its mother, and the other from its father. If one allele is damaged, then the second allele can be used as a reference to correct the damaged strand. In non-homologous end joining, the homologous template is not used during the DNA repair process. Rather, a team of enzymes grabs the broken ends and trims or adds nucleotides as needed to reconnect them. This process tends to be inaccurate and results in deletions or insertions that wind up disabling the gene. In Dr. He’s procedure, this is probably what happened with the twin who had both of her CCR5 receptor genes disabled.
Another DNA repair process is homology-directed repair (HDR). In this process, the homologous allele is used as a template by DNA repair enzymes, which repair the double-stranded break in a way that leaves the gene in a functional state. Possibly, the second twin in Dr. He’s procedure experienced homologous repair (though it’s also possible that the CRISPR-Cas9 only disabled one of her two alleles).
Note that homologous repair opens up the possibility of adding new DNA. In this method, the targeted DNA breakage by CRISPR-Cas9 is followed by the introduction of what’s labeled in figure 7 above as “Donor DNA.” This DNA has ends that are homologous to the broken strand, but with new DNA inserted between the homologous ends. This new DNA could be a working version of a broken gene or a gene from another species. When homologous repair enzymes repair the broken DNA, they’ll wind up inserting this new DNA. This gives genetic engineers a relatively easy way to create recombinant DNA within living cells. In other words, CRISPR-Cas9 allows genetic engineers to cut genes (by non-homologous end joining, and to paste in new genes (by homology-directed repair).
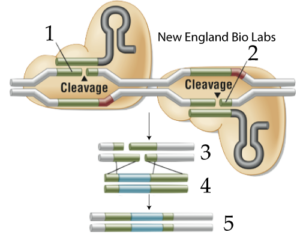
If you want to make sure that your cut will be in the right place (avoiding what’s called “off-target” cleavage), you can use two engineered CRISPR-Cas9s, each of which has been designed to make a single-stranded nick in the target DNA (instead of a double-stranded cut). These nicks are indicated at 1 and 2 in figure 8, at right. If you add donor DNA (shown at 4) to your cut DNA (at 3), then homologous repair mechanisms will join the DNA together, creating engineered recombinant DNA (5).
In short, a combination of sequencing, CRISPR-Cas9, and DNA repair enables researchers to
- Disable genes
- Replace disabled genes with edited genes.
Other variations allow CRISPR-Cas9 to be used to visualize genes and control gene expression.
We’ll look at some applications in a moment. But first, let’s consolidate what you’ve learned.
7. Quiz: CRISPR-Cas9 for Genome Editing
[qwiz random = “true” qrecord_id=”sciencemusicvideosMeister1961-CRISPR 3, Genome Editing (M17)”] [h]
CRISPR-Cas9 as a gene editing system
[i]
[q] In the diagram below, nuclease enzymes are indicated by
[textentry single_char=”true”]
[c]IG I=[Qq]
[f]IEV4Y2VsbGVudCEgTnVjbGVhc2UgZW56eW1lcyBhcmUgaW5kaWNhdGVkIGJ5IHRoZSBzY2lzc29ycyBhdCAmIzgyMjA7Yi4mIzgyMjE7[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBOdWNsZWFzZXMgYXJlIGVuenltZXMgdGhhdCBjdXQgRE5BLiBXaGF0JiM4MjE3O3Mgc29tZXRoaW5nIHlvdSBjdXQgd2l0aD8=[Qq]
[q] In the diagram below, the protospacer is indicated by
[textentry single_char=”true”]
[c]IG M=[Qq]
[f]IEV4Y2VsbGVudCEgVGhlIHByb3Rvc3BhY2VyIGlzIHRoZSB0YXJnZXQgRE5BIHRoYXQmIzgyMTc7cyByZWNvZ25pemVkIGJ5IHRoZSBjclJOQSwgYW5kIGl0JiM4MjE3O3MgaW5kaWNhdGVkIGJ5ICYjODIyMDtjLiYjODIyMTs=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgcHJvdG9zcGFjZXIgaXMgdGhlIEROQSB0aGF0IHdhcyB1c2VkIHRvIGNyZWF0ZSB0aGUgc3BhY2VyLCB3aGljaCBpcyB0aGUgZ2VuZSBmb3IgdGhlIFJOQSB0aGF0IGNvbXBsZW1lbnRzIHdpdGggKGFuZCB0aHVzIHJlY29nbml6ZXMpIEROQSBzZXF1ZW5jZXMgZnJvbSBhdHRhY2tpbmcgYmFjdGVyaW9waGFnZS4=[Qq]
[q] In the diagram below, the short DNA sequence that identifies this DNA as viral DNA that should be cut to prevent a viral takeover of the cell is
[textentry single_char=”true”]
[c]IG Q=[Qq]
[f]IEV4Y2VsbGVudCEgTGV0dGVyICYjODIyMDtkJiM4MjIxOyBpbmRpY2F0ZXMgdGhlIFBBTSwgdGhlIHByb3Rvc3BhY2VyIGFkamFjZW50IG1vdGlmLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBZb3UmIzgyMTc7cmUgbG9va2luZyBmb3IgYSBzaG9ydCBzZXF1ZW5jZSBvZiBETkEgdGhhdCYjODIxNztzIGltbWVkaWF0ZWx5IGFkamFjZW50IHRvIHRoZSBwcm90b3NwYWNlci4gVGhlIHByb3Rvc3BhY2VyIGlzIHdoYXQmIzgyMTc7cyByZWNvZ25pemVkIGJ5ICYjODIyMDtmLCYjODIyMTsgdGhlIGNyUk5BLg==[Qq]
[q] In the diagram below, tracrRNA is indicated by
[textentry single_char=”true”]
[c]IG U=[Qq]
[f]IEV4Y2VsbGVudCEgTGV0dGVyICYjODIyMDtlJiM4MjIxOyBpbmRpY2F0ZXMgdHJhY3JSTkEsIGFuIFJOQSBzZWdtZW50IHJlcXVpcmVkIGZvciBhY3RpdmF0aW5nIHRoZSBDUklTUFItQ2FzOSBjb21wbGV4Lg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiB0cmFjclJOQSBpcyBzb21ldGltZXMgZGVzY3JpYmVkIGFzIGEgaGFuZGxlLg==[Qq]
[q] In the diagram below, sg (single guide) RNA is indicated by
[textentry single_char=”true”]
[c]IG Y=[Qq]
[f]IEV4Y2VsbGVudCEgTGV0dGVyICYjODIyMDtmJiM4MjIxOyBpbmRpY2F0ZXMgc2dSTkEgYW4gZW5naW5lZXJlZCBSTkEgc2VnbWVudCB0aGF0IGxpbmtzIHRvZ2V0aGVyIHRoZSBjclJOQSBhbmQgdGhlIHRyYWNyUk5BIGluIHdpbGQtdHlwZSBDUklTUFItQ2FzOSBzeXN0ZW1zLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBUaGVyZSYjODIxNztzIG9ubHkgb25lIFJOQSBtb2xlY3VsZSBzaG93biBhYm92ZS4gWW91IGNhbiBpZGVudGlmeSBSTkEgYmVjYXVzZSBpdCYjODIxNztzIHNpbmdsZS1zdHJhbmRlZCAoYXMgb3Bwb3NlZCB0byB0aGUgZG91YmxlLXN0cmFuZGVkIEROQSBhdCAmIzgyMjA7YSYjODIyMTspLg==[Qq]
[q multiple_choice=”true”] The CRISPR-Cas9 shown below is
[c]IGVuZ2lu ZWVyZWQ=[Qq]
[f]IEV4Y2VsbGVudC4gWW91IGtub3cgdGhhdCBpdCYjODIxNztzIGVuZ2luZWVyZWQgYmVjYXVzZSB0aGVyZSYjODIxNztzIG9ubHkgb25lIHN0cmFuZCBvZiBSTkE6IGFuIGVuZ2luZWVyZWQgc3RyYW5kIHRoYXQgbGlua3MgdG9nZXRoZXIgdHJhY3JSTkEgYW5kIGNyUk5BLg==[Qq]
[c]IHRoZSAmIzgyMjA7d2lsZC10eXBlJiM4MjIxOyBjb21wbGV4IGZvdW5kIGluIHNwZWNpZXMgbGlrZSA=Uy5weW9nZW5lcy4=[Qq]
[f]IE5vLiBXaWxkLXR5cGUgQ1JJU1BSLUNhczkgaGFzIHR3byBSTkEgbW9sZWN1bGVzLg==[Qq]
[q multiple_choice=”true”] In the diagram below, which number is showing homologous repair?
[c]IDE=[Qq]
[f]IE5vLiBUaGlzIGxvb2tzIG1vcmUgbGlrZSBub24taG9tb2xvZ291cyBlbmQgam9pbmluZyAod2hpY2ggb2Z0ZW4gcmVzdWx0cyBpbiBsb3NzIG9mIGZ1bmN0aW9uIG11dGF0aW9ucywgYW5vdGhlciBjbHVlKS4=[Qq]
[c]ID I=[Qq]
[f]IEV4Y2VsbGVudC4gWW91IGNhbiBzZWUgb25lIEROQSBzdHJhbmQgbGluaW5nIHVwIGFnYWluc3QgYW5vdGhlciBvbmUsIGFuZCB0aGUgYWRkaXRpb24gb2YgZG9ub3IgRE5BLiBCb3RoIGFyZSBjbHVlcyB0aGF0IHRoaXMgcmVwcmVzZW50cyBob21vbG9nb3VzIHJlcGFpci4=[Qq]
[q] Which number in the diagram below represents a process used to cut targeted sequences of DNA?
[textentry single_char=”true”]
[c]ID E=[Qq]
[f]IE5pY2Ugam9iLiAmIzgyMjA7MSYjODIyMTsgaXMgc2hvd2luZyBDUklTUFItQ2FzOSBjdXR0aW5nIGEgdGFyZ2V0IHN0cmFuZCBvZiBETkEu[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBKdXN0IGxvb2sgZm9yIHRoZSBwYXJ0IG9mIHRoZSBkaWFncmFtIHdoZXJlIGEgc3RyYW5kIG9mIEROQSBpcyBiZWluZyBjdXQgaW50byB0d28gZnJhZ21lbnRzLg==[Qq]
[q] Which number shows the process that a researcher might use to silence or deactivate a gene?
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IFRlcnJpZmljLiBUaGUgZGVsZXRpb25zIG9yIGluc2VydGlvbnMgaW5kaWNhdGVkIGJ5ICYjODIyMDsyJiM4MjIxOyByZXByZXNlbnQgbm9uLWhvbW9sb2dvdXMgZW5kIGpvaW5pbmcgcmVwYWlyIG9mIEROQS4gVGhlc2UgdXN1YWxseSByZXN1bHQgaW4gbXV0YXRpb25zIHRoYXQgZGVhY3RpdmF0ZSBnZW5lcy4=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBKdXN0IGxvb2sgZm9yIHRoZSBwYXJ0IG9mIHRoZSBkaWFncmFtIHdoZXJlIGEgc3RyYW5kIG9mIEROQSBpcyBiZWluZyBjdXQgaW50byB0d28gZnJhZ21lbnRzLg==[Qq]
[q] Which number shows the process that a researcher might use to introduce new genes into a cell (or an entire organism)?
[textentry single_char=”true”]
[c]ID M=[Qq]
[f]IFdheSB0byBnby4gTnVtYmVyICYjODIyMDszJiM4MjIxOyByZXByZXNlbnRzIGhvbW9sb2dvdXMgRE5BIHJlcGFpciBhY2NvbXBhbmllZCBieSBpbnNlcnRpb24gb2YgZG9ub3IgRE5BLiBUaGlzIHByb2NlZHVyZSBjYW4gcmVzdWx0IG9mIGluc2VydGlvbiBvZiBuZXcgRE5BIG9yZ2FuaXNtcyBpbnRvIGNlbGxzLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBKdXN0IGxvb2sgZm9yIHRoZSBwYXJ0IG9mIHRoZSBkaWFncmFtIHdoZXJlIGEgc3RyYW5kIG9mIEROQSBpcyBiZWluZyBjdXQgaW50byB0d28gZnJhZ21lbnRzLg==[Qq]
[q] The acronym used to describe the short sequence at “d” is [hangman].
[c]IFBBTQ==[Qq]
[f]IEdyZWF0IQ==[Qq]
[q] Letter “c” is a[hangman] .
[c]IHByb3Rvc3BhY2Vy[Qq]
[f]IENvcnJlY3Qh[Qq]
[q] The two Cas9s shown below have been engineered to create a single-stranded [hangman] at points 1 and 2, instead of a double-stranded cut.
[c]IG5pY2s=[Qq]
[q] The insertion of a new DNA in steps 3, 4, and 5 below is an example of [hangman] directed repair.
[c]IGhvbW9sb2d5[Qq]
[/qwiz]
8. Some CRISPR-Cas9 Applications
Gene editing using CRISPR-Cas9 is progressing at such a pace that by the time you read this the applications listed below will have been superseded by newer ones. Searching for “CRISPR Applications” will get you to the latest developments. In the meanwhile, here’s a starting list of some procedures that biologists are working on…
- Potential therapies for inherited genetic disorders.
- Duchenne Muscular Dystrophy (DMD) is an X-linked, recessive condition that results in the deterioration of muscle tissue, including cardiac (heart) muscle. DMD occurs almost exclusively in boys because girls usually have a second, normal allele on the X chromosome they inherit from their fathers. Boys with this condition rarely live into their thirties. In mice models of DMD, CRISPR was used to target the introns that flank the disease-causing mutation and replace the mutated allele with a functional version, allowing these mice to produce functional muscle tissue (source: Molecular Therapy Nucleic Acids).
- Cystic fibrosis is an autosomal recessive condition related to a mutation in a chloride ion channel protein in cell membranes. The mutation results in membrane channels that are absent, defective, or too few in number. Without the channel, chloride doesn’t flow out of cells, which results in the uptake of water from the extracellular fluid. In mucus-secreting cells, this causes a buildup of thick, sticky mucus, with various effects in different tissues. In the lungs, the result is recurrent bacterial infections. In the digestive tract, it results in poor absorption of nutrients.
In cultured stem cell lines and organoids (3-dimensional tissue cultures used in research) from patients with cystic fibrosis, CRISPR-Cas9 gene-editing combined with homology-directed gene repair was used to remove the mutated allele and replace it with a functioning version. Subsequent experimentation in the repaired organoids showed that the repaired cells were able to process chloride normally (source: Cell: Stem Cell, with an update here). - Sickle Cell Disease is caused by a substitution mutation in the gene for hemoglobin, the oxygen-carrying protein in red blood cells. The mutation results in a single amino acid substitution (with valine inserted in the place of glutamic acid). This changes the chemistry of hemoglobin, causing it to polymerize in low oxygen conditions, creating sickle-shaped red blood cells that clog up capillaries, causing tissue damage and severe pain. One proposed gene editing solution that’s nearing clinical trials would involve isolating stem cells from patients with sickle cell disease, using CRISPR-Cas9 and homology-directed repair to remove the mutated allele and replace it with a working version, and then transplanting the corrected cells back into the patient (source: Human Genetics). Another approach involves using CRISPR-Cas9 to create a mutation that increases the production of fetal hemoglobin (normally turned off in adults) by downregulating a gene called BCL11A, which suppresses the production of fetal hemoglobin after birth. Turning the fetal hemoglobin gene back on can potentially ameliorate sickle cell disease symptoms in children and adults (source: Hematology Times for an overview, or Molecular Therapy for an in-depth review).
- Fighting Viruses: CRISPR-Cas9’s evolutionary roots are as a counterattack against bacteriophage. Hence, it’s no surprise that the system is being used against human viral pathogens. One application being researched is to target HIV when it’s embedded as a provirus in T-cells. Unlike current therapies, which suppress HIV, this therapy could eradicate it from people who are HIV positive (Scientific Reports, volume 8, Article number: 7784 (2018)) Similarly, CRISPR is being investigated as a means to eradicate endogenous retroviruses from pig organs, making them suitable for transplant into humans (sciencemag.org)
- Agriculture: CRISPR works with plant cells as well as it works in animal cells. CRISPR-based gene editing is already being used to increase pest resistance in crops such as wheat, corn, and tomatoes. In addition, CRISPR is being used to introduce new traits into pennycress, a low-value cover crop that’s being engineered for increased biofuel oil production (source: Nature Reviews Molecular Cell Biology, volume19, pages 275–276 (2018)).
- Cancer Research and Therapy
- In some cancers, Killer T cells that would normally attack tumors are deactivated by signals from cancer cells. A planned trial involves using CRISPR-Cas9 to delete two genes in T cells and then infuse them back into the patients, where they’ll be able to seek out and destroy tumors (source: MIT Technology Review).
- The Human Papilloma Virus (HPV) causes genital warts which, if untreated, can progress to cervical cancer. In cervical cancer cell lines (HPV-infected cancer cells that are being grown in cultures), CRISPR-Cas9 targeting of two genes was able to induce these cancerous cells to commit programmed cell suicide, also known as apoptosis. Similar treatments might also be possible for other virally induced cancers, such as Burkitt’s lymphoma, which can be caused by the Epstein Barr Virus (source: Molecular Therapy).
For further Reading about CRISPR and its potential applications (from three of its discoverers), read
The Kavli Prize Interview with Jennifer Doudna, Emmanuelle Charpentier, and Virginijus Šikšnys.
9. CRISPR-Cas9: Cumulative Quiz
The quiz below will test your understanding of CRISPR-Cas9 Biology.
[qwiz random = “true” style=”width: 600px !important;” qrecord_id=”sciencemusicvideosMeister1961-CRISPR 4, cumulative quiz (M17)”] [h]
CRISPR-Cas9, Cumulative Quiz
[i]
[q] DNA that originally came from a virus is shown at
[textentry single_char=”true”]
[c]IE M=[Qq]
[f]IEV4Y2VsbGVudC4gVGhlIHNwYWNlciBETkEsIHNob3duIGF0IFM=MQ==LCBTMg==LCBldGMuIGlzIGhvdyBiYWN0ZXJpYSAmIzgyMjA7cmVtZW1iZXImIzgyMjE7IHByZXZpb3VzIHZpcmFsIGF0dGFja2Vycy4=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlIEROQSBvZiB2aXJhbCBvcmlnaW4gdGhhdCYjODIxNztzIGluc2VydGVkIGludG8gdGhlIENSSVNQUiBhcnJheSBpcyBjYWxsZWQgYSAmIzgyMjA7c3BhY2VyLiYjODIyMTs=[Qq]
[q] Spacer DNA is shown at
[textentry single_char=”true”]
[c]IE M=[Qq]
[f]IEV4Y2VsbGVudC4gVGhlIHNwYWNlciBETkEgaXMgc2hvd24gYXQgUw==MQ==LCBTMg==LCBldGMuIFRoZSBzcGFjZXJzIGFyZSBob3cgYmFjdGVyaWEgJiM4MjIwO3JlbWVtYmVyJiM4MjIxOyBwcmV2aW91cyB2aXJhbCBhdHRhY2tlcnMu[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLsKg[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlIHNwYWNlciBETkEgaXMgaW50ZXJzcGVyc2VkIHdpdGggcmVwZWF0cy7CoA==[Qq]
[q] In the diagram below, a variety of viral DNA fragments are shown at
[textentry single_char=”true”]
[c]IG U=[Qq]
[f]IEdvb2QhIEZyYWdtZW50ZWQgdmlyYWwgRE5BIGlzIHNob3duIGF0ICYjODIyMDtlLiYjODIyMTs=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlIEROQSBpbnNlcnRlZCBieSB0aGUgdmlydXMgaXMgc2hvd24gYXQgJiM4MjIwO2MuJiM4MjIxOyBJZiB0aGF0IEROQSB3ZXJlIGZyYWdtZW50ZWQsIHdoYXQgd291bGQgaXQgbG9vayBsaWtlP8Kg[Qq]
[q] In the diagram below, the viral DNA that will form the new spacer is shown at
[textentry single_char=”true”]
[c]IG Y=[Qq]
[f]IEdvb2QhIFRoZSB2aXJhbCBETkEgZnJhZ21lbnQgdGhhdCB3aWxsIGJlY29tZSBhIG5ldyBzcGFjZXIgaXMgc2hvd24gYXQgJiM4MjIwO2YuJiM4MjIxO8Kg[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBJbiB0aGUgZGlhZ3JhbSwgZmluZCB0aGUgRE5BIGZyYWdtZW50IHRoYXQmIzgyMTc7cyBnb2luZyB0byBiZSBpbnNlcnRlZCBpbnRvIHRoZSBDUklTUFIgYXJyYXkgKGF0ICYjODIyMDtHJiM4MjIxOykuwqA=[Qq]
[q] In the diagram below, the CRISPR array is shown at
[textentry single_char=”true”]
[c]IG o=[Qq]
[f]IENvcnJlY3QhIFRoZSBDUklTUFIgYXJyYXkgY29uc2lzdHMgb2Ygc3BhY2VycyAoaSkgYW5kIHJlcGVhdHMgKGgpLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IFNvcnJ5LCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlwqBDUklTUFIgYXJyYXkgY29uc2lzdHMgb2Ygc3BhY2VycyAoaSkgYW5kIHJlcGVhdHMgKGgpLiBTZWUgaWYgeW91IGNhbiBjb25uZWN0IHRoZSBkaWFncmFtIGJlbG93IHRvIHRoZSBvbmUgYWJvdmUuwqA=
Cg==[Qq]
[q] In the diagram below, the only letter that points to the Cas9 protein before it has associated with RNA is
[textentry single_char=”true”]
[c]IE o=[Qq]
[f]IEV4Y2VsbGVudCEgJiM4MjIwO0omIzgyMjE7IHNob3dzIHRoZSBDYXM5IHByb3RlaW4gYmVmb3JlIGl0IGhhcyBhc3NvY2lhdGVkIHdpdGggYW55IFJOQXMuwqA=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gTGV0dGVyICYjODIyMDtvJiM4MjIxOyBzaG93cyBDUklTUFItQ2FzOS4gV2hlcmUgY2FuIHlvdSBmaW5kIHRoZSBDYXM5IHdpdGhvdXQgYW55IFJOQT8=[Qq]
[q] In the diagram below, pre-CRISPR RNA is shown at
[textentry single_char=”true”]
[c]IG c=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7ZyYjODIyMTsgc2hvd3MgcHJlLUNSSVNQUiBSTkEu[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gTGV0dGVyICYjODIyMDtmJiM4MjIxOyBzaG93cyBDUklTUFIgRE5BLg==[Qq]
[q] In the diagram below, crRNA is shown at
[textentry single_char=”true”]
[c]IH A=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7cCYjODIyMTsgc2hvd3MgY3JSTkEuIFlvdSBjYW4gdGVsbCBiZWNhdXNlIGl0JiM4MjE3O3MgYmluZGluZyB3aXRoIHZpcmFsIEROQS7CoA==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gTG9vayBmb3Igc2luZ2xlLXN0cmFuZGVkIFJOQSB0aGF0JiM4MjE3O3MgYmluZGluZyB3aXRoIHZpcmFsIEROQS7CoA==[Qq]
[q] In the diagram below, viral DNA that’s been cleaved by Cas9 is shown at
[textentry single_char=”true”]
[c]IH E=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7cSYjODIyMTsgc2hvd3MgY2xlYXZlZCB2aXJhbCBETkEuwqA=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVmlyYWwgRE5BIGlzIGdvaW5nIHRvIGJlIGZyYWdtZW50ZWQgYWZ0ZXI=IGl0JiM4MjE3O3MgYWN0ZWQgdXBvbiBieSBDUklTUFItQ2FzOS4=[Qq]
[q] In the diagram below, mature CRISPR-Cas9 is shown at
[textentry single_char=”true”]
[c]IG 8=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7byYjODIyMTsgc2hvd3MgbWF0dXJlIENSSVNQUi1DYXM5[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gQ1JJU1BSLUNhczksIG9uY2UgbWF0dXJlLCBjYW4gZmluZCBhbmQgZnJhZ21lbnQgdmlyYWwgRE5BIHdpdGggYSBwcm90b3NwYWNlciB0aGF0IGNvbXBsZW1lbnRzIGl0cyBjclJOQS4=[Qq]
[q] In the diagram below, the protospacer would be found within
[textentry single_char=”true”]
[c]IG M=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7YyYjODIyMTsgc2hvd3MgdmlyYWwgRE5BLiBXaXRoaW4gdGhhdCBETkEgc2VxdWVuY2UgaXMgYSBwcm90b3NwYWNlciB0aGF0IENSSVNQUi1DYXM5IGNhbiBiaW5kIHdpdGggYW5kIGNsZWF2ZSBhcGFydC7CoA==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlIHByb3Rvc3BhY2VyIGlzIGEgc2VxdWVuY2Ugd2l0aGluIHRoZSB2aXJhbCBETkEuIEZpbmQgdGhlIHZpcmFsIEROQSwgYW5kIHlvdSYjODIxNztsbCBzZWUgd2hlcmUgdGhlIHByb3Rvc3BhY2VyIGhhcyB0byBiZS7CoA==[Qq]
[q] In the diagram below, the CRISPR array is found at
[textentry single_char=”true”]
[c]IG M=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7YyYjODIyMTsgc2hvd3MgdGhlIENSSVNQUiBhcnJheS4=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gWW91JiM4MjE3O3JlIGxvb2tpbmcgZm9yIGFuIGFycmF5IG9mIHJlcGVhdHMgYW5kIHNwYWNlcnMuwqA=[Qq]
[q] In the diagram below, spacers are indicated by which letter? (Note: don’t worry about the subscript)
[textentry single_char=”true”]
[c]IG I=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7YiYjODIyMTsgaW5kaWNhdGVzIHRoZSBzcGFjZXJzLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlIHNwYWNlcnMgYXJlIHBhcnQgb2YgdGhlIENSSVNQUiBhcnJheSwgYW5kIHRoZXkmIzgyMTc7cmUgc2VwYXJhdGVkIGJ5IGlkZW50aWNhbCByZXBlYXQgc2VxdWVuY2VzLsKg[Qq]
[q] In the diagram below, the bacterial chromosome is indicated by
[textentry single_char=”true”]
[c]IG Q=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7ZCYjODIyMTsgaW5kaWNhdGVzIHRoZSBiYWN0ZXJpYWwgY2hyb21vc29tZS7CoA==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlIGJhY3RlcmlhbCBjaHJvbW9zb21lIGlzIGEgY2lyY3VsYXIgcGllY2Ugb2YgRE5BIHRoYXQgaW5jbHVkZXMgdGhlIENSSVNQUiBhcnJheSwgQ1JJU1BSLWFzc29jaWF0ZWQgZ2VuZXMsIGFuZCBhbGwgb2YgdGhlIGJhY3RlcmlhbCBjZWxsJiM4MjE3O3Mgb3RoZXIgZ2VuZXMuwqA=[Qq]
[q] In the diagram below, pre-crRNA is indicated by
[textentry single_char=”true”]
[c]IG U=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7ZSYjODIyMTsgaW5kaWNhdGVzIHByZS1jclJOQS7CoA==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gcHJlLWNyUk5BIHdpbGwgYmUgdHJhbnNjcmliZWQgZnJvbSB0aGUgQ1JJU1BSIEROQSwgd2hpY2ggaXMgc2hvd24gYXQgJiM4MjIwO2MuJiM4MjIxOw==[Qq]
[q] In the diagram below, crRNA that hasn’t yet associated with the Cas9 protein is shown at
[textentry single_char=”true”]
[c]IG Y=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7ZiYjODIyMTsgaW5kaWNhdGVzIGNyUk5BLsKg[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gQXQgc3RhZ2UgMywgeW91IGNhbiBzZWUgZm91ciBDUklTUFItQ2FzOXMuIEVhY2ggb2YgdGhlc2UgY29uc2lzdHMgb2YgYSBDYXM5IHByb3RlaW4gKGluIGJsdWUpIGFzc29jaWF0ZWQgd2l0aCBhIGNyUk5BLg==[Qq]
[q] In the diagram below, a protospacer is shown at which letter? Note: don’t worry about using a subscript in your answer.
[textentry single_char=”true”]
[c]IG g=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7aCYjODIyMTsgaW5kaWNhdGVzIHRoZSBwcm90b3NwYWNlci4gVGhpcyBpcyB0aGUgdmlyYWwgRE5BIHNlcXVlbmNlIHRoYXQgY29tcGxlbWVudHMgdGhlIGNyUk5BLCBhbmQgd2hpY2ggaXMgdHJhbnNjcmliZWQgZnJvbSB0aGUgc3BhY2VyLsKg[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlIHByb3Rvc3BhY2VyIGlzIHZpcmFsIEROQSB0aGF0JiM4MjE3O3MgY29tcGxlbWVudGFyeSB0byBjclJOQS4=[Qq]
[q] In the diagram below, the PAM is shown by which letter? Note: don’t worry about using a subscript in your answer.
[textentry single_char=”true”]
[c]IG k=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7aSYjODIyMTsgaW5kaWNhdGVzIHRoZSBQQU06IHRoZSBwcm90b3NwYWNlciBhZGphY2VudCBtb3RpZi4=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlIFBBTSBpcyBhbiBhY3JvbnltIGZvciA=cHJvdG9zcGFjZXIgYWRqYWNlbnQgbW90aWY=LiBJdCYjODIxNztzIGFkamFjZW50IHRvIHRoZSBwcm90b3NwYWNlciwgd2hpY2ggaXMgYSByZWNvZ25pemVkIHNlcXVlbmNlIHdpdGhpbiB0aGUgdmlyYWwgRE5BLg==[Qq]
[q] In the diagram below, the PAM is shown by which letter?
[textentry single_char=”true”]
[c]IG I=[Qq]
[f]IEF3ZXNvbWUhIFRoZSBsZXR0ZXIgJiM4MjIwO2kmIzgyMjE7IGluZGljYXRlcyB0aGUgUEFNOiB0aGUgcHJvdG9zcGFjZXIgYWRqYWNlbnQgbW90aWYu[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlIFBBTSBpcyBhbiBhY3JvbnltIGZvciA=cHJvdG9zcGFjZXIgYWRqYWNlbnQgbW90aWY=LiBJdCYjODIxNztzIGFkamFjZW50IHRvIHRoZSBwcm90b3NwYWNlciwgd2hpY2ggaXMgYSByZWNvZ25pemVkIHNlcXVlbmNlIHdpdGhpbiB0aGUgdmlyYWwgRE5BLg==[Qq]
[q] In the diagram below, which short sequence of DNA bases acts as a signal to Cas9 saying “it’s ok to cleave this DNA.”
[textentry single_char=”true”]
[c]IG I=[Qq]
[f]IEF3ZXNvbWUhIFRoZSBsZXR0ZXIgJiM4MjIwO2kmIzgyMjE7IGluZGljYXRlcyB0aGUgUEFNOiB0aGUgcHJvdG9zcGFjZXIgYWRqYWNlbnQgbW90aWYu[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gWW91JiM4MjE3O3JlIGxvb2tpbmcgZm9yIHRoZSBwcm90b3NwYWNlciBhZGphY2VudCBtb3RpZiBvciBQQU0uIEZpbmQgc29tZXRoaW5nIHRoYXQmIzgyMTc7cyBhZGphY2VudCB0byB0aGUgcHJvdG9zcGFjZXIu[Qq]
[q] In the diagram below, the sgRNA is indicated by
[textentry single_char=”true”]
[c]IG E=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7YSYjODIyMTsgaW5kaWNhdGVzIHRoZSBzZ1JOQSwgYW4gZW5naW5lZXJlZCBSTkEgdGhhdCBmdXNlcyB0b2dldGhlciBjclJOQSBhbmQgdHJhY3JSTkEu[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gUk5BIGlzIHNpbmdsZS1zdHJhbmRlZC4=[Qq]
[q] In the diagram below, the protospacer is indicated by
[textentry single_char=”true”]
[c]IG M=[Qq]
[f]IEF3ZXNvbWUhIExldHRlciAmIzgyMjA7YyYjODIyMTsgaW5kaWNhdGVzIHRoZSBwcm90b3NwYWNlciwgdGhlIHNlcXVlbmNlIG9mIEROQSB0aGF0IENSSVNQUi1DYXM5IGhhcyBiZWVuIHByb2dyYW1tZWQgdG8gc2VlayBvdXQgYW5kIGNsZWF2ZS7CoA==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLCBidXQgaGVyZSYjODIxNztzIGEgaGludC4gVGhlwqA=cHJvdG9zcGFjZXIgaXMgdGhlIHNlcXVlbmNlIG9mIEROQSB0aGF0IENSSVNQUi1DYXM5IGhhcyBiZWVuIHByb2dyYW1tZWQgdG8gc2VlayBvdXQgYW5kIGNsZWF2ZS7CoA==[Qq]
[q] In the diagram below, non-homologous end joining is indicated by
[textentry single_char=”true”]
[c]IG Q=[Qq]
[f]IFRlcnJpZmljISBMZXR0ZXIgJiM4MjIwO2QmIzgyMjE7IGluZGljYXRlcyBub24taG9tb2xvZ291cyBlbmQgam9pbmluZy7CoA==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBMb29rIGZvciBhIHBhcnQgb2YgdGhlIGRpYWdyYW0gaW4gd2hpY2ggdGhlIGVuZHMgb2YgdGhlIEROQSBhcmUgYmVpbmcgc3RpdGNoZWQgdG9nZXRoZXIsIHdpdGhvdXQgcmVmZXJlbmNlIHRvIGFueSBvdGhlciBzdHJhbmQgb2YgRE5BLsKg[Qq]
[q] In the diagram below, which letter indicates a process that could be used to deactivate/knock out a gene?
[textentry single_char=”true”]
[c]IG Q=[Qq]
[f]IFRlcnJpZmljISBMZXR0ZXIgJiM4MjIwO2QmIzgyMjE7IGluZGljYXRlcyBub24taG9tb2xvZ291cyBlbmQgam9pbmluZy4gVGhlIG11dGF0aW9ucyB0aGF0IGFyZSBhc3NvY2lhdGVkIHdpdGggdGhhdCBwcm9jZXNzIHVzdWFsbHkgd2luZCB1cCBkZWFjdGl2YXRpbmcgb3Iga25vY2tpbmcgb3V0IGdlbmUgYWN0aXZpdHkuwqA=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBMb29rIGZvciBhIHBhcnQgb2YgdGhlIGRpYWdyYW0gaW4gd2hpY2ggdGhlIGVuZHMgb2YgdGhlIEROQSBhcmUgYmVpbmcgc3RpdGNoZWQgdG9nZXRoZXIsIHdpdGhvdXQgcmVmZXJlbmNlIHRvIGFueSBvdGhlciBzdHJhbmQgb2YgRE5BLsKg[Qq]
[q] In the diagram below, which letter indicates a process that could be used to insert a new gene?
[textentry single_char=”true”]
[c]IG U=[Qq]
[f]IFRlcnJpZmljISBMZXR0ZXIgJiM4MjIwO2UmIzgyMjE7IGluZGljYXRlcyBob21vbG9neS1kaXJlY3RlZCByZXBhaXIuIFRoZSBwYXJ0IG9mIHRoZSBzZXF1ZW5jZSBpbmRpY2F0ZWQgYnkgdGhlIHJlZCByZWN0YW5nbGUgaW5kaWNhdGVzIHRoZSBpbnNlcnRpb24gb2YgbmV3IEROQS7CoA==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBMb29rIGZvciBhIHBhcnQgb2YgdGhlIGRpYWdyYW0gaW4gd2hpY2ggdGhlIEROQSBpcyByZXBhaXJlZCBieSB1c2luZyBhIGhvbW9sb2dvdXMgdGVtcGxhdGUgYXMgYSByZWZlcmVuY2UuwqA=[Qq]
[/qwiz]
10. My Interview with Jennifer Doudna
In April, 2020, I interviewed Professor Doudna as part of a virtual fundraising luncheon for the Berkeley High School Development Group. Enjoy!
11. Links
At least for now, this tutorial ends this module on biotechnology and genetic engineering. If you have ideas for other topics, please email me.
