Click the following link for a Carbon and Functional Groups Student Learning Guide to accompany this tutorial.
1. Introduction
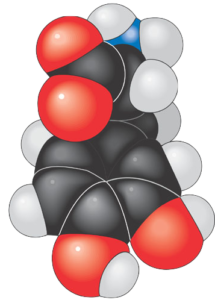
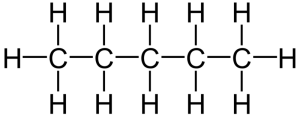
In the last tutorial, we saw how carbon can covalently bond with itself and atoms of other elements to form chains, rings, and branched molecules. Carbon’s versatility in forming bonds allows for molecules that have the same number and types of atoms, but which have different structures. For example, the two molecules below are both variations of the formula C5H12. That makes them isomers: molecules with the same number and types of atoms, but with different structures. Once we know a bit more chemistry, we’ll come back to isomers and look at some of their biological implications.
| Chain | Branched molecules |
 |
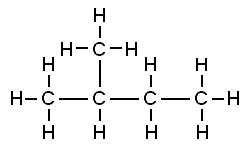 |
Because the two molecules above consist solely of carbon and hydrogen, they’re classified as hydrocarbons. Fossil fuels (petroleum, natural gas, and coal) are hydrocarbons. For over 200 years, fossil fuels have powered the rise of our industrialized civilization. At the same time, emissions from burning fossil fuels are disrupting our climate, a topic you can learn more about at our Greenhouse Effect and Climate Disruption tutorial.
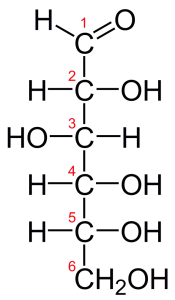
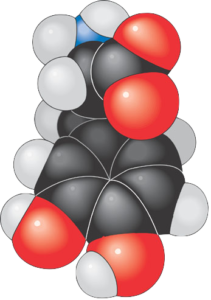
While hydrocarbons are essential fuels, they’re not particularly important in living things. Rather, the molecules that compose living things are typically organized like the molecule of glucose (a common sugar) shown to the right. What you see, in this case, is a chain of carbon atoms, with many of the carbons attached to small groups of other atoms. These small groups of atoms act as a unit and are called functional groups.
Let’s continue using glucose as an example. If you look at carbon number 1, you can see that this carbon atom, in addition to sharing electrons with a hydrogen atom, is also double-bonded to oxygen. That carbon, with its double bond to oxygen, is a functional group, and its presence (along with other functional groups) helps to determine the chemistry of whatever molecule it’s attached to. Similarly, Carbons 2 through six are bonded both to hydrogen and to an -OH. The “-OH” is another functional group.
Biology students need to know seven of these groups. Start by taking a few minutes to study the table below. If you are unfamiliar with the idea of polarity and its consequences, you can jump back to our tutorials on water, polarity, and hydrogen bonding.
| Name | Structure | Key effect on molecules |
| Hydroxyl | Makes a molecule polar. | |
| Carbonyl | 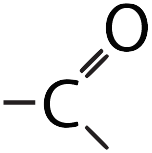 |
Makes a molecule polar. |
| Carboxyl | 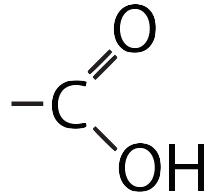 |
Makes a molecule acidic (because it can donate H+ to a solution). |
| Amino | 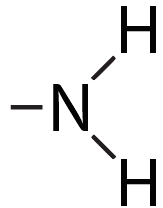 |
Makes a molecule basic (because it picks up an H+ from the solution). |
| Sulfhydryl | Two sulfhydryls can form Sulfur-Sulfur bonds (also called “disulfide bridges”), which are important in protein structure. | |
| Phosphate | 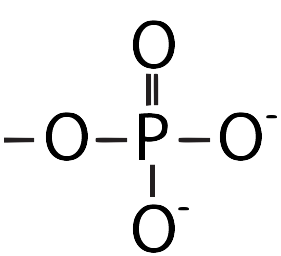 |
Important in energy transfer. |
| Methyl | 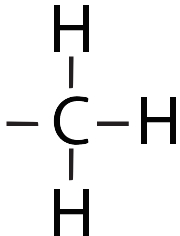 |
Makes a molecule non-polar. Can bind to DNA, affecting gene activity (usually turning genes “off”) |
| Acetyl | 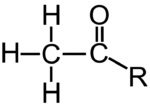 |
A component of many organic molecules. Can bind to DNA-associated proteins, enhancing gene expression. |
2. Quiz: Identifying Functional groups
[qwiz random = “true” qrecord_id=”sciencemusicvideosMeister1961-Functional Groups Quiz 1 (M5)”]
[h]Quiz: Identifying Functional Groups
[i]The following quiz will show you the structural formulas of each of the functional groups. Some of the questions will ask you to write in the name, hangman style. Others questions will be multiple choice.
[q]This functional group is
[c]aHlkcm94eW wgwqAgwqA=[Qq][c]Y2FyYm9ueWwgwqAgwqDCoA==[Qq][c]Y2FyYm94eWwgwqDCoA==[Qq][c]YW1pbm8=
Cg==[Qq][c]c3VsZmh5ZHJ5bCDCoMKg[Qq][c]cGhvc3BoYXRlIMKgwqA=[Qq][c]bWV0aHlsIA==[Qq][c]YWNldHls
Cg==[Qq][f]WWVzLiBBIGh5ZHJveHlsIGdyb3VwIGNvbnNpc3RzIG9mIG94eWdlbiBib25kZWQgdG8gaHlkcm9nZW4s
Cg==[Qq]
[f]Tm8uIEEgY2FyYm9ueWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[f]Tm8uIEEgQ2FyYm94eWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhbmQgc2luZ2xlIGJvbmRlZCB0byBhIGh5ZHJveHlsIGdyb3VwICgtT0gp
Cg==[Qq]
[f]Tm8uIEFuIGFtaW5vIGdyb3VwIGNvbnNpc3RzIG9mIGEgbml0cm9nZW4gYXRvbSB3aXRoIHNpbmdsZSBib25kcyB0byB0d28gaHlkcm9nZW4gYXRvbXMu
Cg==[Qq]
[f]Tm8uIEEgc3VsZmh5ZHJ5bCBncm91cCBjb25zaXN0cyBvZiBhIHN1bGZ1ciBhdG9tIGJvbmRlZCB0byBhIGh5ZHJvZ2VuIGF0b20u
Cg==[Qq]
[f]Tm8uIEEgcGhvc3BoYXRlIGdyb3VwIGNvbnNpc3RzIG9mIGEgcGhvc3Bob3J1cyBhdG9tIHN1cnJvdW5kZWQgYnkgZm91ciBveHlnZW5zLiBUaGUgcGhvc3Bob3J1cyBzaGFyZXMgYSBkb3VibGUgYm9uZCB3aXRoIG9uZSBvZiB0aGUgb3h5Z2VucyBhbmQgYSBzaW5nbGUgYm9uZCB3aXRoIGVhY2ggb2YgdGhlIG90aGVyIHRocmVlLiBUd28gb2YgdGhlIG94eWdlbnMgaGF2ZSBhIG5lZ2F0aXZlIGNoYXJnZS4=
Cg==[Qq]
[f]Tm8uIEEgbWV0aHlsIGdyb3VwIGNvbnNpc3RzIG9mIGEgY2FyYm9uIHNpbmdsZS1ib25kZWQgd2l0aCB0aHJlZSBoeWRyb2dlbnMu
Cg==[Qq]
[f]Tm8uIEFuIGFjZXR5bCBncm91cCBjb25zaXN0cyBvZiBhIG1ldGh5bCBncm91cCBjb25uZWN0ZWQgdG8gYSBjYXJib255bCBncm91cC4=
Cg==Cg==[Qq]
[q]This functional group is
[c]aHlkcm94eWwgwqAgwqA=[Qq][c]Y2FyYm9ueWwg wqAgwqDCoA==[Qq][c]Y2FyYm94eWwgwqDCoA==[Qq][c]YW1pbm8=
Cg==[Qq][c]c3VsZmh5ZHJ5bCDCoMKg[Qq][c]cGhvc3BoYXRlIMKgwqA=[Qq][c]bWV0aHlswqAgwqA=[Qq][c]YWNldHls
Cg==[Qq][f]Tm8uIEEgaHlkcm94eWwgZ3JvdXAgY29uc2lzdHMgb2Ygb3h5Z2VuIGJvbmRlZCB0byBoeWRyb2dlbiw=
Cg==[Qq]
[f]WWVzLiBBIGNhcmJvbnlsIGdyb3VwIGhhcyBhIGNhcmJvbiBhdG9tIHRoYXQmIzgyMTc7cyBkb3VibGUtYm9uZGVkIHRvIG94eWdlbiwgYXMgc2hvd24gYmVsb3cu
Cg==[Qq]
[f]Tm8uIEEgQ2FyYm94eWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhbmQgc2luZ2xlIGJvbmRlZCB0byBhIGh5ZHJveHlsIGdyb3VwICgtT0gp
Cg==[Qq]
[f]Tm8uIEFuIGFtaW5vIGdyb3VwIGNvbnNpc3RzIG9mIGEgbml0cm9nZW4gYXRvbSB3aXRoIHNpbmdsZSBib25kcyB0byB0d28gaHlkcm9nZW4gYXRvbXMu
Cg==[Qq]
[f]Tm8uIEEgc3VsZmh5ZHJ5bCBncm91cCBjb25zaXN0cyBvZiBhIHN1bGZ1ciBhdG9tIGJvbmRlZCB0byBhIGh5ZHJvZ2VuIGF0b20u
Cg==[Qq]
[f]Tm8uIEEgcGhvc3BoYXRlIGdyb3VwIGNvbnNpc3RzIG9mIGEgcGhvc3Bob3J1cyBhdG9tIHN1cnJvdW5kZWQgYnkgZm91ciBveHlnZW5zLiBUaGUgcGhvc3Bob3J1cyBzaGFyZXMgYSBkb3VibGUgYm9uZCB3aXRoIG9uZSBvZiB0aGUgb3h5Z2VucyBhbmQgYSBzaW5nbGUgYm9uZCB3aXRoIGVhY2ggb2YgdGhlIG90aGVyIHRocmVlLiBUd28gb2YgdGhlIG94eWdlbnMgaGF2ZSBhIG5lZ2F0aXZlIGNoYXJnZS4=
Cg==[Qq]
[f]Tm8uIEEgbWV0aHlsIGdyb3VwIGNvbnNpc3RzIG9mIGEgY2FyYm9uIHNpbmdsZS1ib25kZWQgd2l0aCB0aHJlZSBoeWRyb2dlbnMu
Cg==[Qq]
[f]Tm8uIEFuIGFjZXR5bCBncm91cCBjb25zaXN0cyBvZiBhIG1ldGh5bCBncm91cCBjb25uZWN0ZWQgdG8gYSBjYXJib255bCBncm91cC4=
Cg==Cg==[Qq]
[q]This functional group is
[c]aHlkcm94eWwgwqAgwqA=[Qq][c]Y2FyYm9ueWwgwqAgwqDCoA==[Qq][c]Y2FyYm94eW wgwqDCoA==[Qq][c]YW1pbm8=
Cg==[Qq][c]c3VsZmh5ZHJ5bCDCoMKg[Qq][c]cGhvc3BoYXRlIMKgwqA=[Qq][c]bWV0aHlswqA=[Qq][c]YWNldHls
Cg==[Qq][f]Tm8uIEEgaHlkcm94eWwgZ3JvdXAgY29uc2lzdHMgb2Ygb3h5Z2VuIGJvbmRlZCB0byBoeWRyb2dlbiw=
Cg==[Qq]
[f]Tm8uIEEgY2FyYm9ueWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[f]WWVzLiBBIENhcmJveHlsIGdyb3VwIGhhcyBhIGNhcmJvbiBhdG9tIHRoYXQmIzgyMTc7cyBkb3VibGUtYm9uZGVkIHRvIG94eWdlbiBhbmQgc2luZ2xlIGJvbmRlZCB0byBhIGh5ZHJveHlsIGdyb3VwICgtT0gp
Cg==[Qq]
[f]Tm8uIEFuIGFtaW5vIGdyb3VwIGNvbnNpc3RzIG9mIGEgbml0cm9nZW4gYXRvbSB3aXRoIHNpbmdsZSBib25kcyB0byB0d28gaHlkcm9nZW4gYXRvbXMu
Cg==[Qq]
[f]Tm8uIEEgc3VsZmh5ZHJ5bCBncm91cCBjb25zaXN0cyBvZiBhIHN1bGZ1ciBhdG9tIGJvbmRlZCB0byBhIGh5ZHJvZ2VuIGF0b20u
Cg==[Qq]
[f]Tm8uIEEgcGhvc3BoYXRlIGdyb3VwIGNvbnNpc3RzIG9mIGEgcGhvc3Bob3J1cyBhdG9tIHN1cnJvdW5kZWQgYnkgZm91ciBveHlnZW5zLiBUaGUgcGhvc3Bob3J1cyBzaGFyZXMgYSBkb3VibGUgYm9uZCB3aXRoIG9uZSBvZiB0aGUgb3h5Z2VucyBhbmQgYSBzaW5nbGUgYm9uZCB3aXRoIGVhY2ggb2YgdGhlIG90aGVyIHRocmVlLiBUd28gb2YgdGhlIG94eWdlbnMgaGF2ZSBhIG5lZ2F0aXZlIGNoYXJnZS4=
Cg==[Qq]
[f]Tm8uIEEgbWV0aHlsIGdyb3VwIGNvbnNpc3RzIG9mIGEgY2FyYm9uIHNpbmdsZS1ib25kZWQgd2l0aCB0aHJlZSBoeWRyb2dlbnMu
Cg==[Qq]
[f]Tm8uIEFuIGFjZXR5bCBncm91cCBjb25zaXN0cyBvZiBhIG1ldGh5bCBncm91cCBjb25uZWN0ZWQgdG8gYSBjYXJib255bCBncm91cC4=
Cg==Cg==[Qq]
[q]This functional group is
[c]aHlkcm94eWwgwqAgwqA=[Qq][c]Y2FyYm9ueWwgwqAgwqDCoA==[Qq][c]Y2FyYm94eWwgwqDCoA==[Qq][c]YW1p bm8=
Cg==[Qq][c]c3VsZmh5ZHJ5bCDCoMKg[Qq][c]cGhvc3BoYXRlIMKgwqA=[Qq][c]bWV0aHlswqA=[Qq][c]YWNldHls
Cg==[Qq][f]Tm8uIEEgaHlkcm94eWwgZ3JvdXAgY29uc2lzdHMgb2Ygb3h5Z2VuIGJvbmRlZCB0byBoeWRyb2dlbiw=
Cg==[Qq]
[f]Tm8uIEEgY2FyYm9ueWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[f]Tm8uIEEgQ2FyYm94eWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhbmQgc2luZ2xlIGJvbmRlZCB0byBhIGh5ZHJveHlsIGdyb3VwICgtT0gp
Cg==[Qq]
[f]WWVzLiBBbiBhbWlubyBncm91cCBjb25zaXN0cyBvZiBhIG5pdHJvZ2VuIGF0b20gd2l0aCBzaW5nbGUgYm9uZHMgdG8gdHdvIGh5ZHJvZ2VuIGF0b21zLg==
Cg==[Qq]
[f]Tm8uIEEgc3VsZmh5ZHJ5bCBncm91cCBjb25zaXN0cyBvZiBhIHN1bGZ1ciBhdG9tIGJvbmRlZCB0byBhIGh5ZHJvZ2VuIGF0b20u
Cg==[Qq]
[f]Tm8uIEEgcGhvc3BoYXRlIGdyb3VwIGNvbnNpc3RzIG9mIGEgcGhvc3Bob3J1cyBhdG9tIHN1cnJvdW5kZWQgYnkgZm91ciBveHlnZW5zLiBUaGUgcGhvc3Bob3J1cyBzaGFyZXMgYSBkb3VibGUgYm9uZCB3aXRoIG9uZSBvZiB0aGUgb3h5Z2VucyBhbmQgYSBzaW5nbGUgYm9uZCB3aXRoIGVhY2ggb2YgdGhlIG90aGVyIHRocmVlLiBUd28gb2YgdGhlIG94eWdlbnMgaGF2ZSBhIG5lZ2F0aXZlIGNoYXJnZS4=
Cg==[Qq]
[f]Tm8uIEEgbWV0aHlsIGdyb3VwIGNvbnNpc3RzIG9mIGEgY2FyYm9uIHNpbmdsZS1ib25kZWQgd2l0aCB0aHJlZSBoeWRyb2dlbnMu
Cg==[Qq]
[f]Tm8uIEFuIGFjZXR5bCBncm91cCBjb25zaXN0cyBvZiBhIG1ldGh5bCBncm91cCBjb25uZWN0ZWQgdG8gYSBjYXJib255bCBncm91cC4=
Cg==Cg==[Qq]
[q]This functional group is
[c]aHlkcm94eWwgwqAgwqA=[Qq][c]Y2FyYm9ueWwgwqAgwqDCoA==[Qq][c]Y2FyYm94eWwgwqDCoA==[Qq][c]YW1pbm8=
Cg==[Qq][c]c3VsZmh5ZH J5bCDCoMKg[Qq][c]cGhvc3BoYXRlIMKgwqA=[Qq][c]bWV0aHlswqA=[Qq][c]YWNldHls
Cg==[Qq][f]Tm8uIEEgaHlkcm94eWwgZ3JvdXAgY29uc2lzdHMgb2Ygb3h5Z2VuIGJvbmRlZCB0byBoeWRyb2dlbiw=
Cg==[Qq]
[f]Tm8uIEEgY2FyYm9ueWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[f]Tm8uIEEgQ2FyYm94eWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhbmQgc2luZ2xlIGJvbmRlZCB0byBhIGh5ZHJveHlsIGdyb3VwICgtT0gp
Cg==[Qq]
[f]Tm8uIEFuIGFtaW5vIGdyb3VwIGNvbnNpc3RzIG9mIGEgbml0cm9nZW4gYXRvbSB3aXRoIHNpbmdsZSBib25kcyB0byB0d28gaHlkcm9nZW4gYXRvbXMu
Cg==[Qq]
[f]WWVzLiBBIHN1bGZoeWRyeWwgZ3JvdXAgY29uc2lzdHMgb2YgYSBzdWxmdXIgYXRvbSBib25kZWQgdG8gYSBoeWRyb2dlbiBhdG9tLg==
Cg==[Qq]
[f]Tm8uIEEgcGhvc3BoYXRlIGdyb3VwIGNvbnNpc3RzIG9mIGEgcGhvc3Bob3J1cyBhdG9tIHN1cnJvdW5kZWQgYnkgZm91ciBveHlnZW5zLiBUaGUgcGhvc3Bob3J1cyBzaGFyZXMgYSBkb3VibGUgYm9uZCB3aXRoIG9uZSBvZiB0aGUgb3h5Z2VucyBhbmQgYSBzaW5nbGUgYm9uZCB3aXRoIGVhY2ggb2YgdGhlIG90aGVyIHRocmVlLiBUd28gb2YgdGhlIG94eWdlbnMgaGF2ZSBhIG5lZ2F0aXZlIGNoYXJnZS4=
Cg==[Qq]
[f]Tm8uIEEgbWV0aHlsIGdyb3VwIGNvbnNpc3RzIG9mIGEgY2FyYm9uIHNpbmdsZS1ib25kZWQgd2l0aCB0aHJlZSBoeWRyb2dlbnMu
Cg==[Qq]
[f]Tm8uIEFuIGFjZXR5bCBncm91cCBjb25zaXN0cyBvZiBhIG1ldGh5bCBncm91cCBjb25uZWN0ZWQgdG8gYSBjYXJib255bCBncm91cC4=
Cg==Cg==[Qq]
[q]This functional group is
[c]aHlkcm94eWwgwqAgwqA=[Qq][c]Y2FyYm9ueWwgwqAgwqDCoA==[Qq][c]Y2FyYm94eWwgwqDCoA==[Qq][c]YW1pbm8=
Cg==[Qq][c]c3VsZmh5ZHJ5bCDCoMKg[Qq][c]cGhvc3BoYX RlIMKgwqA=[Qq][c]bWV0aHlswqA=[Qq][c]YWNldHls
Cg==[Qq][f]Tm8uIEEgaHlkcm94eWwgZ3JvdXAgY29uc2lzdHMgb2Ygb3h5Z2VuIGJvbmRlZCB0byBoeWRyb2dlbiw=
Cg==[Qq]
[f]Tm8uIEEgY2FyYm9ueWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[f]Tm8uIEEgQ2FyYm94eWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhbmQgc2luZ2xlIGJvbmRlZCB0byBhIGh5ZHJveHlsIGdyb3VwICgtT0gp
Cg==[Qq]
[f]Tm8uIEFuIGFtaW5vIGdyb3VwIGNvbnNpc3RzIG9mIGEgbml0cm9nZW4gYXRvbSB3aXRoIHNpbmdsZSBib25kcyB0byB0d28gaHlkcm9nZW4gYXRvbXMu
Cg==[Qq]
[f]Tm8uIEEgc3VsZmh5ZHJ5bCBncm91cCBjb25zaXN0cyBvZiBhIHN1bGZ1ciBhdG9tIGJvbmRlZCB0byBhIGh5ZHJvZ2VuIGF0b20u
Cg==[Qq]
[f]WWVzLiBBIHBob3NwaGF0ZSBncm91cCBjb25zaXN0cyBvZiBhIHBob3NwaG9ydXMgYXRvbSBzdXJyb3VuZGVkIGJ5IGZvdXIgb3h5Z2Vucy4gVGhlIHBob3NwaG9ydXMgc2hhcmVzIGEgZG91YmxlIGJvbmQgd2l0aCBvbmUgb2YgdGhlIG94eWdlbnMgYW5kIGEgc2luZ2xlIGJvbmQgd2l0aCBlYWNoIG9mIHRoZSBvdGhlciB0aHJlZS4gVHdvIG9mIHRoZSBveHlnZW5zIGhhdmUgYSBuZWdhdGl2ZSBjaGFyZ2Uu
Cg==[Qq]
[f]Tm8uIEEgbWV0aHlsIGdyb3VwIGNvbnNpc3RzIG9mIGEgY2FyYm9uIHNpbmdsZS1ib25kZWQgd2l0aCB0aHJlZSBoeWRyb2dlbnMu
Cg==[Qq]
[f]Tm8uIEFuIGFjZXR5bCBncm91cCBjb25zaXN0cyBvZiBhIG1ldGh5bCBncm91cCBjb25uZWN0ZWQgdG8gYSBjYXJib255bCBncm91cC4=
Cg==Cg==[Qq]
[q]This functional group is
[c]aHlkcm94eWwgwqAgwqA=[Qq][c]Y2FyYm9ueWwgwqAgwqDCoA==[Qq][c]Y2FyYm94eWwgwqDCoA==[Qq][c]YW1pbm8=
Cg==[Qq][c]c3VsZmh5ZHJ5bCDCoMKg[Qq][c]cGhvc3BoYXRlIMKgwqA=[Qq][c]bWV0aH lswqA=[Qq][c]YWNldHls
Cg==[Qq][f]WWVzLiBBIGh5ZHJveHlsIGdyb3VwIGNvbnNpc3RzIG9mIG94eWdlbiBib25kZWQgdG8gaHlkcm9nZW4s
Cg==[Qq]
[f]Tm8uIEEgY2FyYm9ueWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[f]Tm8uIEEgQ2FyYm94eWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuIGFuZCBzaW5nbGUtYm9uZGVkIHRvIGEgaHlkcm94eWwgZ3JvdXAgKC1PSCk=
Cg==[Qq]
[f]Tm8uIEFuIGFtaW5vIGdyb3VwIGNvbnNpc3RzIG9mIGEgbml0cm9nZW4gYXRvbSB3aXRoIHNpbmdsZSBib25kcyB0byB0d28gaHlkcm9nZW4gYXRvbXMu
Cg==[Qq]
[f]Tm8uIEEgc3VsZmh5ZHJ5bCBncm91cCBjb25zaXN0cyBvZiBhIHN1bGZ1ciBhdG9tIGJvbmRlZCB0byBhIGh5ZHJvZ2VuIGF0b20u
Cg==[Qq]
[f]Tm8uIEEgcGhvc3BoYXRlIGdyb3VwIGNvbnNpc3RzIG9mIGEgcGhvc3Bob3J1cyBhdG9tIHN1cnJvdW5kZWQgYnkgZm91ciBveHlnZW5zLiBUaGUgcGhvc3Bob3J1cyBzaGFyZXMgYSBkb3VibGUgYm9uZCB3aXRoIG9uZSBvZiB0aGUgb3h5Z2VucyBhbmQgYSBzaW5nbGUgYm9uZCB3aXRoIGVhY2ggb2YgdGhlIG90aGVyIHRocmVlLiBUd28gb2YgdGhlIG94eWdlbnMgaGF2ZSBhIG5lZ2F0aXZlIGNoYXJnZS4=
Cg==[Qq]
[f]WWVzLiBBIG1ldGh5bCBncm91cCBjb25zaXN0cyBvZiBhIGNhcmJvbiBzaW5nbGUtYm9uZGVkIHdpdGggdGhyZWUgaHlkcm9nZW5zLg==
Cg==[Qq]
[f]Tm8uIEFuIGFjZXR5bCBncm91cCBjb25zaXN0cyBvZiBhIG1ldGh5bCBncm91cCBjb25uZWN0ZWQgdG8gYSBjYXJib255bCBncm91cC4=
Cg==Cg==[Qq]
[q]This functional group is
[c]aHlkcm94eWwgwqAgwqA=[Qq][c]Y2FyYm9ueWwgwqAgwqDCoA==[Qq][c]Y2FyYm94eWwgwqDCoA==[Qq][c]YW1pbm8=
Cg==[Qq][c]c3VsZmh5ZHJ5bCDCoMKg[Qq][c]cGhvc3BoYXRlIMKgwqA=[Qq][c]bWV0aHlswqA=[Qq][c]YWNl dHls
Cg==[Qq][f]WWVzLiBBIGh5ZHJveHlsIGdyb3VwIGNvbnNpc3RzIG9mIG94eWdlbiBib25kZWQgdG8gaHlkcm9nZW4sIGFzIHNob3duIGJlbG93Lg==
Cg==[Qq]
[f]Tm8uIEEgY2FyYm9ueWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhcyBzaG93biBiZWxvdy4=
Cg==[Qq]
[f]Tm8uIEEgQ2FyYm94eWwgZ3JvdXAgaGFzIGEgY2FyYm9uIGF0b20gdGhhdCYjODIxNztzIGRvdWJsZS1ib25kZWQgdG8gb3h5Z2VuLCBhbmQgc2luZ2xlIGJvbmRlZCB0byBhIGh5ZHJveHlsIGdyb3VwICgtT0gp
Cg==[Qq]
[f]Tm8uIEFuIGFtaW5vIGdyb3VwIGNvbnNpc3RzIG9mIGEgbml0cm9nZW4gYXRvbSB3aXRoIHNpbmdsZSBib25kcyB0byB0d28gaHlkcm9nZW4gYXRvbXMu
Cg==[Qq]
[f]Tm8uIEEgc3VsZmh5ZHJ5bCBncm91cCBjb25zaXN0cyBvZiBhIHN1bGZ1ciBhdG9tIGJvbmRlZCB0byBhIGh5ZHJvZ2VuIGF0b20u
Cg==[Qq]
[f]Tm8uIEEgcGhvc3BoYXRlIGdyb3VwIGNvbnNpc3RzIG9mIGEgcGhvc3Bob3J1cyBhdG9tIHN1cnJvdW5kZWQgYnkgZm91ciBveHlnZW5zLiBUaGUgcGhvc3Bob3J1cyBzaGFyZXMgYSBkb3VibGUgYm9uZCB3aXRoIG9uZSBvZiB0aGUgb3h5Z2VucyBhbmQgYSBzaW5nbGUgYm9uZCB3aXRoIGVhY2ggb2YgdGhlIG90aGVyIHRocmVlLiBUd28gb2YgdGhlIG94eWdlbnMgaGF2ZSBhIG5lZ2F0aXZlIGNoYXJnZS4=
Cg==[Qq]
[f]WWVzLiBBIG1ldGh5bCBncm91cCBjb25zaXN0cyBvZiBhIGNhcmJvbiBzaW5nbGUtYm9uZGVkIHdpdGggdGhyZWUgaHlkcm9nZW5zLg==
Cg==[Qq]
[f]RmFidWxvdXMuIEFuIGFjZXR5bCBncm91cCBjb25zaXN0cyBvZiBhIG1ldGh5bCBncm91cCBjb25uZWN0ZWQgdG8gYSBjYXJib255bCBncm91cC4=
Cg==Cg==[Qq]
[q]This functional group is
[hangman]
[c]aHlkcm 94eWw=[Qq]
[f]WWVzLiBUaGUgbmFtZSBvZiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgaHlkcm94eWw=Lg==
Cg==[Qq]
[q]This functional group is
[hangman]
[c]Y2FyYm 9ueWw=[Qq]
[f]WWVzLiBUaGUgbmFtZSBvZiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgY2FyYm9ueWw=Lg==
Cg==[Qq]
[q]This functional group is
[hangman]
[c]Y2FyYm 94eWw=[Qq]
[f]WWVzLiBUaGUgbmFtZSBvZiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgY2FyYm94eWw=Lg==
Cg==[Qq]
[q]This functional group is
[hangman]
[c]YW1p bm8=[Qq]
[f]WWVzLiBUaGUgbmFtZSBvZiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgYW1pbm8=Lg==
Cgo=[Qq]
[q]This functional group is
[hangman]
[c]c3VsZmh5 ZHJ5bA==[Qq]
[f]WWVzLiBUaGUgbmFtZSBvZiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgc3VsZmh5ZHJ5bA==Lg==
Cg==[Qq]
[q]This functional group is
[hangman]
[c]cGhvc3 BoYXRl[Qq]
[f]WWVzLiBUaGUgbmFtZSBvZiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgcGhvc3BoYXRlLg==
Cg==[Qq]
[q]This functional group is
[hangman]
[c]bWV0 aHls[Qq]
[f]WWVzLiBUaGUgbmFtZSBvZiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgbWV0aHlsLg==
Cg==[Qq]
[q]This functional group is
[hangman]
[c]YWNl dHls[Qq]
[f]WWVzLiBUaGUgbmFtZSBvZiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgYWNldHlsLg==[Qq]
[x]
[restart]
[/qwiz]
3. Ionized functional groups
Three of the functional groups, carboxyl, amino, and phosphate can be shown in an ionized or unionized form. You should be able to recognize both forms when you see them attached to molecules. Study the table below.
| Name | Non-ionized form | Ionized form |
| Carboxyl |  |
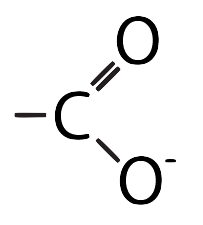 |
| Amino |  |
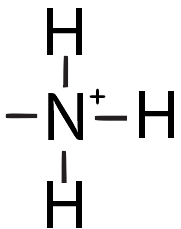 |
| Phosphate | 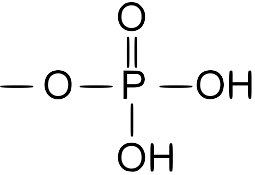 |
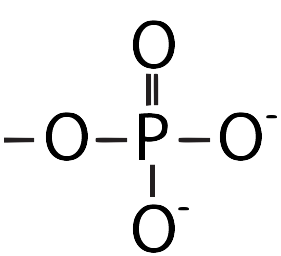 |
4. More about Isomers
At the beginning of this tutorial, I described how molecules with the same number and types of atoms, but different arrangements of those atoms, are called isomers. There are three types of isomers to know about.
Structural isomers are the kind we looked at above (and shown again below for your convenience).
| Chain | Branched molecules |
 |
 |
Both of these molecules have the same molecular formula (C5H12), but their structural arrangement differs. The different arrangements can have the effect of giving these molecules different physical and chemical properties.
The second type of isomer is a cis-trans isomer. Cis-trans isomers are the result of the fact that double bonds (two shared pairs of electrons) are geometrically fixed, and don’t allow the atoms they join to rotate around the bond axis. Take a look at these two molecules, both of which have the formula C4H8.
| C4H8, cis configuration | C4H8, trans configuration |
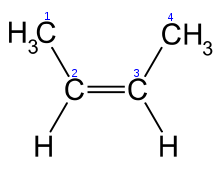 |
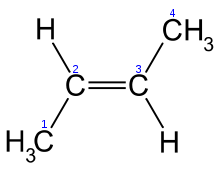 |
They are clearly isomers, with a different geometry. Note how the two methyl groups in the molecule on the left are on the same side of the double bond, which is referred to as a cis configuration. In the molecule on the right, the two methyl groups are on opposite sides of the double bond, which is referred to as a trans configuration. As with structural isomers, the different locations of the methyl groups can alter the chemical and physical properties of these molecules.
You might have heard about trans fats, and how they’re bad for your heart. That’s correct, and you’ll learn more about them in my videos and interactive tutorials about biochemistry. Click here if you want to jump ahead to that topic now.
The last type of isomers are molecules that are non-superimposable mirror images of one another. That sounds abstract, but it’s simple. Hold your hands out in front of you. Your hands are analogous to enantiomers: same bones, same digits, with a mirror image structure. If you made a mold that perfectly fit your right hand, your left hand wouldn’t be able to fit into it. These types of isomers are called enantiomers.
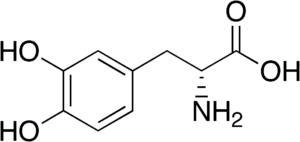
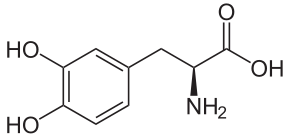
The two molecules below are both chemical relatives of the neurotransmitter dopamine.
| D-dopa (biologically inactive) | L-dopa (biologically active) |
 |
 |
|
|
|
Neurotransmitters are the chemicals used to send signals from one nerve cell to the next. Dopamine works in the brains of animals (including humans) in a variety of processes, including reward and movement regulation.
D-dopa and L-dopa look pretty similar, right? Let’s focus on the structural formulas. Remember that in this type of structural formula, every angle vertex indicates a carbon atom. Note that the amino group in D-dopa is shown as being attached to its carbon with a dashed wedge. That dashed wedge means that the amino group is below the plane of the molecule. In L-dopa, there’s a solid wedge connecting the amino group to its carbon. That solid wedge indicates that the amino group is above the plane of the molecule.
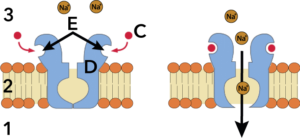
That difference might seem trivial, but it makes these molecules as different (and as non-interchangeable) as your left and right hands. That’s because neurotransmitters (represented by “3” on the right) work by binding with receptors (E) on cells. In the same way that you can’t put a right-handed glove on your left hand, a receptor that can bind with L-dopa can’t bind with D-dopa. The consequence is that L-dopa can serve as a medicine for people with Parkinson’s disease, a condition where the brain produces insufficient amounts of dopamine. D-dopa has no pharmacological use.
5. Another Quiz: Functional groups and isomers
That’s about all you need to know about carbon and functional groups to succeed in an AP Bio or a first-year, introductory biology course. When we study proteins (as well as other key biological molecules) in the next module of our course, you’ll see that it will be useful to be able to identify functional groups and to hold in mind which ones are polar, non-polar, acidic, basic, and so on. In the quiz that follows, just to keep you on your toes, some of the functional groups shown below are in their ionized form, but others are not. Enjoy!
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Functional Groups Quiz 2 (M5)”]
[h]Functional groups and isomers
[i]
[q]In the diagram below, a hydroxyl group is shown at
[textentry single_char=”true”]
[c]ID U=[Qq]
[f]IE5pY2Ugam9iOiBhIGh5ZHJveHlsIGdyb3VwIGlzIGF0ICYjODIyMDs1LiYjODIyMTs=[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgaHlkcm94eWwgZ3JvdXAgaXMgbGlrZSBhIHdhdGVyIG1vbGVjdWxlLCBidXQgaXQmIzgyMTc7cyBtaXNzaW5nIGEgaHlkcm9nZW4gYXRvbS4=
Cg==[Qq]
[q]In the diagram below, a methyl group is shown at
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IE5pY2Ugam9iOiBhIG1ldGh5bMKgZ3JvdXAgaXMgYXQgJiM4MjIwOzIuJiM4MjIxOw==[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgbWV0aHlswqBncm91cCBpcyBsaWtlIGEgbWV0aGFuZcKgbW9sZWN1bGUsIChDSA==NA==KSwgYnV0IGl0JiM4MjE3O3MgbWlzc2luZyBhIGh5ZHJvZ2VuIGF0b20u
Cg==[Qq]
[q]In the diagram below, a phosphate group is shown at
[textentry single_char=”true”]
[c]wq Ax[Qq]
[f]IE5pY2Ugam9iOiBhIHBob3NwaGF0ZcKgZ3JvdXAgaXMgYXQgJiM4MjIwOzEuJiM4MjIxOw==[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgcGhvc3BoYXRlwqBncm91cCBpcyB0aGUgb25seSBvbmUgd2l0aCBwaG9zcGhvcnVzLg==
Cg==[Qq]
[q]In the diagram below, a sulfhydryl group is shown at
[textentry single_char=”true”]
[c]wq A2[Qq]
[f]IE5pY2Ugam9iOiBhIHN1bGZoeWRyeWzCoGdyb3VwIGlzIGF0ICYjODIyMDs2LiYjODIyMTs=[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBMb29rIGF0IHRoZSBuYW1lIG9mIHRoaXMgZnVuY3Rpb25hbCBncm91cCAoJiM4MjIwO3N1bGZoeWRyeWwmIzgyMjE7KS4gVGhlIGZpcnN0IHN5bGxhYmxlIHRlbGxzIHlvdSB3aGljaCBlbGVtZW50IHRvIGxvb2sgZm9yLg==
Cg==[Qq]
[q]In the diagram below, a carboxyl group is shown at
[textentry single_char=”true”]
[c]wq Az[Qq]
[f]IE5pY2Ugam9iOiBhIGNhcmJveHlswqBncm91cCBpcyBhdCAmIzgyMjA7My4mIzgyMjE7[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlIGFyZSB0d28gaGludHMuIDEpIFRoZSBuYW1lICYjODIyMDtjYXJib3h5bCYjODIyMTsgaW5kaWNhdGVzIG9uZSBvZiB0aGUga2V5IGVsZW1lbnRzIGluIHRoaXMgZ3JvdXAuIDIpIFRoZSBjYXJib3h5bCBncm91cCBpcyBvbmUgb2YgdGhlIGZ1bmN0aW9uYWwgZ3JvdXBzIHRoYXQgY2FuIGJlIGluIGFuIGlvbml6ZWQgZm9ybSAobWVhbmluZyB0aGF0IGl0IHdpbGwgaGF2ZSBhIGNoYXJnZSAoaW5kaWNhdGVkIGJ5IGEgJiM4MjIwO3BsdXMmIzgyMjE7IG9yIGEgJiM4MjIwO21pbnVzJiM4MjIxOyBzaWduKS4gU28sIGxvb2sgZm9yIHRoZSBlbGVtZW50IEkmIzgyMTc7dmUgaGludGVkIGF0LCBhbmQgYSBjaGFyZ2Uu
Cg==[Qq]
[q]In the diagram below, a carbonyl group is shown at
[textentry single_char=”true”]
[c]wq A3[Qq]
[f]IE5pY2Ugam9iOiBhIGNhcmJvbnlswqBncm91cCBpcyBhdCAmIzgyMjA7Ny4mIzgyMjE7[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgY2FyYm9ueWzCoGdyb3VwJiM4MjE3O3MgbmFtZSBpbmRpY2F0ZXMgb25lIG9mIGl0cyBtb3N0IGltcG9ydGFudCBlbGVtZW50cy4=
Cg==[Qq]
[q]In the diagram below, an amino group is shown at
[textentry single_char=”true”]
[c]wq A0[Qq]
[f]IE5pY2Ugam9iOiBhbiBhbWlubyBncm91cCBpcyBhdCAmIzgyMjA7NC4mIzgyMjE7[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGXCoGZvdXJ0aCBsZXR0ZXIgaW4gdGhlIHdvcmQgJiM4MjIwO2FtaW5vJiM4MjIxOyBpbmRpY2F0ZXMgYSBrZXkgZWxlbWVudCBpbiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAu
Cg==[Qq]
[q]In the diagram below, acetyl is shown at
[textentry single_char=”true”]
[c]wq A4[Qq]
[f]IE5pY2Ugam9iOiBhbiBhY2V0eWwgZ3JvdXAgaXMgYXQgJiM4MjIwOzguJiM4MjIxOw==[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBBY2V0eWwgZ3JvdXBzIGhhdmUgYSBtZXRoeWwgZ3JvdXAgYW5kIGEgY2FyYm9ueWwgZ3JvdXAu
Cg==[Qq]
[q]In the diagram below, the functional group that’s used to deactivate stretches of DNA (turning genes off) is at
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IE5pY2Ugam9iOiBhIG1ldGh5bMKgZ3JvdXAgaXMgYXQgJiM4MjIwOzIuJiM4MjIxOyBUaGVzZSBncm91cHMgYXJlIG9mdGVuIHVzZWQgaW4gZ2VuZSBpbmFjdGl2YXRpb24u[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgVGhpcyBwcm9jZXNzIG9mIHR1cm5pbmcgZ2VuZXMgb2ZmIGJ5IGFkZGluZyBhIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgY2FsbGVkICYjODIyMDttZXRoeWxhdGlvbi4mIzgyMjE7
Cg==[Qq]
[q]In the diagram below, the functional group that’s often used in energy transfer is at
[textentry single_char=”true”]
[c]wq Ax[Qq]
[f]IE5pY2Ugam9iOiBhIHBob3NwaGF0ZcKgZ3JvdXAgaXMgc2hvd24gYXQgJiM4MjIwOzEuJiM4MjIxOyBUaGVzZSBhcmUgb2Z0ZW4gdXNlZCBpbiBlbmVyZ3kgdHJhbnNmZXIgKGFzIGluIHRoZSBtb2xlY3VsZSBhZGVub3NpbmUgdHJpcGhvc3BoYXRlLCBBVFApLg==[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGVzZSB0eXBlcyBvZiBlbmVyZ3kgdHJhbnNmZXJzIGFyZSByZWZlcnJlZCB0byBhcyA=cGhvc3Bob3J5bGF0aW9ucw==Lg==
Cg==[Qq]
[q]In the diagram below, the functional group that plays a key role in protein structure through the formation of disulfide bridges is
[textentry single_char=”true”]
[c]wq A2[Qq]
[f]IE5pY2Ugam9iOiBhIHN1bGZoeWRyeWzCoGdyb3VwIGlzIHNob3duIGF0ICYjODIyMDs2LiYjODIyMTsgVHdvIHN1bGZoeWRyeWwgZ3JvdXBzIGNhbiBmb3JtIGEgc3RydWN0dXJlIGNhbGxlZCBhICYjODIyMDtkaXN1bGZpZGUmIzgyMjE7IGJyaWRnZSwgd2hpY2ggY2FuIGJlbmQgYSBwcm90ZWluIGludG8gYSBwYXJ0aWN1bGFyIHNoYXBlICh3aGljaCBoZWxwcyB0byBkZXRlcm1pbmUgdGhlIHByb3RlaW4mIzgyMTc7cyBzaGFwZSwgd2hpY2ggaW4gdHVybiBoZWxwcyBkZXRlcm1pbmUgaXRzIGZ1bmN0aW9uKS4=[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgVGhlIHN0cnVjdHVyZSBpcyBjYWxsZWQgYSAmIzgyMjA7ZGlzdWxmaWRlIGJyaWRnZS4mIzgyMjE7IFdoYXQgZWxlbWVudCBuYW1lIGRvIHlvdSBzZWUgaW4gJiM4MjIwO2Rpc3VsZmlkZS4mIzgyMjE7IFdoaWNoIGZ1bmN0aW9uYWwgZ3JvdXAgaGFzIHRoYXQgZWxlbWVudD8=
Cg==[Qq]
[q]Note that in this question, the diagram is just for reference. The two functional groups that can make the molecule they’re attached to acidic are
[c]bWV0aHlsIGFuZCBjYXJib255bA==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgTG9vayBmb3IgdGhlIHR3byBmdW5jdGlvbmFsIGdyb3VwcyB0aGF0LCBpbiB0aGVpciBpb25pemVkIGZvcm0sIGhhdmUgbmVnYXRpdmUgY2hhcmdlcy4gVGhleSYjODIxNztyZSBuZWdhdGl2ZSBiZWNhdXNlIHRoZXkgbG9zdCBhIGh5ZHJvZ2VuIGlvbiwgd2hpY2ggaGFzIGRpc3NvbHZlZCBpbnRvIHRoZSBzdXJyb3VuZGluZyBzb2x1dGlvbiwgbWFraW5nIHRoYXQgc29sdXRpb24gbW9yZSBhY2lkaWMu[Qq]
[c]wqBwaG9zcGhhdGUgYW5kIGFtaW5v[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgTG9vayBmb3IgdGhlIHR3byBmdW5jdGlvbmFsIGdyb3VwcyB0aGF0LCBpbiB0aGVpciBpb25pemVkIGZvcm0sIGhhdmUgbmVnYXRpdmUgY2hhcmdlcy4gVGhleSYjODIxNztyZSBuZWdhdGl2ZSBiZWNhdXNlIHRoZXkgbG9zdCBhIGh5ZHJvZ2VuIGlvbiwgd2hpY2ggaGFzIGRpc3NvbHZlZCBpbnRvIHRoZSBzdXJyb3VuZGluZyBzb2x1dGlvbiwgbWFraW5nIHRoYXQgc29sdXRpb24gbW9yZSBhY2lkaWMu[Qq]
[c]c3VsZmh5ZHJ5bMKgYW5kIGNhcmJveHls[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgTG9vayBmb3IgdGhlIHR3byBmdW5jdGlvbmFsIGdyb3VwcyB0aGF0LCBpbiB0aGVpciBpb25pemVkIGZvcm0sIGhhdmUgbmVnYXRpdmUgY2hhcmdlcy4gVGhleSYjODIxNztyZSBuZWdhdGl2ZSBiZWNhdXNlIHRoZXkgbG9zdCBhIGh5ZHJvZ2VuIGlvbiwgd2hpY2ggaGFzIGRpc3NvbHZlZCBpbnRvIHRoZSBzdXJyb3VuZGluZyBzb2x1dGlvbiwgbWFraW5nIHRoYXQgc29sdXRpb24gbW9yZSBhY2lkaWMu[Qq]
[c]Y2FyYm94eWwgYW5k IHBob3NwaGF0ZQ==[Qq]
[f]IE5pY2Ugam9iOiBUaGUgdHdvIGZ1bmN0aW9uYWwgZ3JvdXBzIHRoYXQgbWFrZSB0aGUgbW9sZWN1bGUgdGhleSYjODIxNztyZSBhdHRhY2hlZCB0byBhY2lkaWMgYXJlIHBob3NwaGF0ZSBhbmQgY2FyYm94eWw=
Cg==Jm5ic3A7
Cg==[Qq]
[q]In the diagram below, the functional group can make the molecule it’s attached to a base is
[textentry single_char=”true”]
[c]wq A0[Qq]
[f]IE5pY2Ugam9iOsKgdGhlIGFtaW5vIGdyb3VwLCBhdCA0LCBjYW4gYWJzb3JiIGEgaHlkcm9nZW4gaW9uIGZyb20gdGhlIHNvbHV0aW9uIGl0JiM4MjE3O3MgaW4sIG1ha2luZyB0aGUgc29sdXRpb24gbW9yZSBiYXNpYy4=[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgQW1tb25pYSBpcyBhIGJhc2UgdGhhdCBoYXMgYSBzdHJ1Y3R1cmUgcmVsYXRlZCB0byB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAuIEFtbW9uaWEmIzgyMTc7cyBmaWZ0aCBsZXR0ZXIgaGFzIGFuIGVsZW1lbnQgdGhhdCB3aWxsIGxlYWQgeW91IHJpZ2h0IHRvIHRoaXMgZnVuY3Rpb25hbCBncm91cC4=
Cg==[Qq]
[q]A general term for two or more molecules with the same molecular formula but a different arrangement of the atoms in these molecules is
[hangman]
[c]aXNv bWVy[Qq]
[f]QSBnZW5lcmFsIHRlcm0gZm9yIHR3byBvciBtb3JlIG1vbGVjdWxlcyB3aXRoIHRoZSBzYW1lIG1vbGVjdWxhciBmb3JtdWxhIGJ1dCBhIGRpZmZlcmVudCBhcnJhbmdlbWVudCBvZiB0aGUgYXRvbXMgaW4gdGhlc2UgbW9sZWN1bGVzIGlzIGFuIA==aXNvbWVyLg==
Cg==[Qq]
[q]Two molecules that are non-superimposable mirror images of one another are known as
[hangman]
[c]ZW5hbnRp b21lcnM=[Qq]
[f]VHdvIG1vbGVjdWxlcyB0aGF0IGFyZSBub24tc3VwZXJpbXBvc2FibGUgbWlycm9yIGltYWdlcyBvZiBvbmUgYW5vdGhlciBhcmUga25vd24gYXMgZW5hbnRpb21lcnMuwqA=
Cg==[Qq]
[q]Cis-trans isomers are molecules with the same number and type of atoms, but with a different arrangement of these atoms around a __________ bond.
[hangman]
[c]ZG91 Ymxl[Qq]
[f]Q2lzLXRyYW5zIGlzb21lcnMgYXJlIG1vbGVjdWxlcyB3aXRoIHRoZSBzYW1lIG51bWJlciBhbmQgdHlwZSBvZiBhdG9tcywgYnV0IHdpdGggZGlmZmVyZW50IGFycmFuZ2VtZW50cyBvZiB0aGVzZSBhdG9tcyBhcm91bmQgYSA=ZG91YmxlwqBib25kLg==
Cg==[Qq]
[q]In the molecule below, the functional group attached to carbon number 1 is a _________ group.
[hangman]
[c]Y2FyYm 9ueWw=[Qq]
[f]RXhjZWxsZW50LiBUaGUgZnVuY3Rpb25hbCBncm91cCBhdHRhY2hlZCB0byBjYXJib24gbnVtYmVyIDEgaXMgYSA=Y2FyYm9ueWw=IGdyb3VwLg==
Cg==[Qq]
[q]In the molecule below, the functional group attached to carbon number 4 is a _________ group.
[hangman]
[c]aHlkcm 94eWw=[Qq]
[f]TmljZS4gVGhlIGZ1bmN0aW9uYWwgZ3JvdXAgYXR0YWNoZWQgdG8gY2FyYm9uIG51bWJlciA0IGlzIGEgaHlkcm94eWw=wqBncm91cC4=
Cg==[Qq]
[q]In the molecule below, the functional group attached to the left side of the central carbon is a(n) [hangman] group (note: the central carbon has the R1 hanging below it: you’ll learn what that is very soon).
[c]YW1p bm8=
Cg==[Qq]
[q]In the molecule below, the functional group attached to the right side of the central carbon is a(n) [hangman] group (note: the central carbon has the R1 hanging below it: you’ll learn what that is very soon).
[c]Y2FyYm 94eWw=
Cg==[Qq]
[q]In the molecule below, there are three of the same functional groups in a row, indicated by the letters “A,” “B,” and “C.” This is a [hangman] group.
[c]cGhvc3 BoYXRl[Qq]
[/qwiz]
6. What now?
Link to
- Proceed to Biochemistry (Carbohydrates, Lipids, Proteins, and Nucleic Acids)
- return to the Carbon and functional groups menu