Click the following link for a Carbon and Functional Groups Student Learning Guide to accompany this tutorial.
1. Why the molecules of life are built around carbon
 Life is complex. Carbon allows for that complexity at the molecular level. Here’s why.
Life is complex. Carbon allows for that complexity at the molecular level. Here’s why.
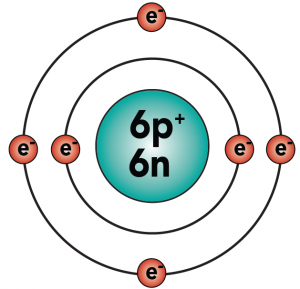
Carbon is element number six in the periodic table. It’s number six because it has six protons, along with six neutrons and six electrons.
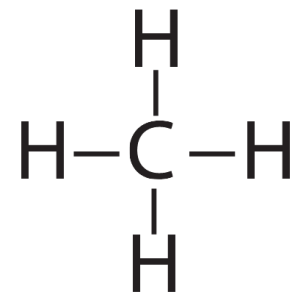
With four outer (also known as valence) electrons, carbon readily forms covalent bonds with other atoms, including other carbon atoms.
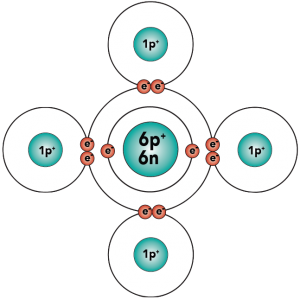
Electron distribution model |
structural formula |
 |
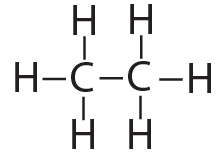 |
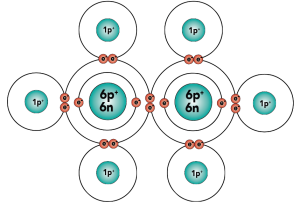
That carbon-carbon sharing makes it possible for carbon to form chains, branched molecules, and rings.
| Chain | Branched molecules | Rings |
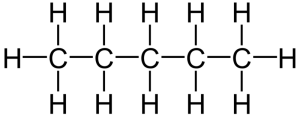 |
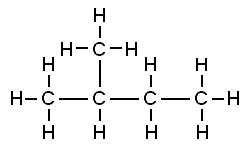 |
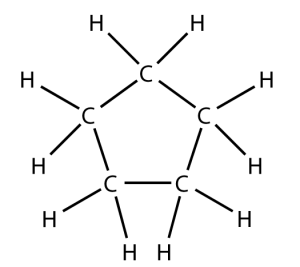 |
The possibilities for complexity and variety are further enhanced by carbon’s ability to form double bonds and triple bonds, as shown below…
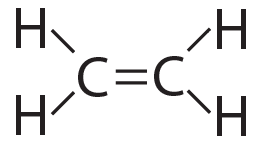
Ethylene (CH4) has a double bond |
Acetylene (CH2) has a triple bond |
So why is this important? The ability of carbon to form bonds with other atoms, particularly with itself, sets the stage for the evolution of the complex molecules that make life possible. Living things do phenomenally complex things, such as taking in matter and energy from the environment to grow and develop, and then reproducing and passing on genes to the next generation. On the molecular level, a variety of “molecular machines” are required to carry out these tasks, and these machines are big molecules, built around a carbon backbone. The flexibility of carbon-based molecules allows for the complexity of life. In the next tutorial, we’ll start seeing how the molecules of life are constructed. But before that, let’s check to make sure that you’re clear on what’s above.
2. Checking Understanding: Carbon and the Molecules of Life
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Carbon and the Molecules of Life (M5)”]
[h] Carbon and the Molecules of Life
[i]
[q] Carbon has _____ protons
[textentry single_char=”true”]
[c]ID Y=[Qq]
[f]IFllcy4gQ2FyYm9uIGhhcyA2IHByb3RvbnMu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBCdXQgeW91IGNhbiBmaWd1cmUgaXQgb3V0IGJ5IGV4YW1pbmluZyB0aGlzIGRpYWdyYW0u[Qq]
[q] Carbon has _______ outer (or valence) electrons.
[textentry single_char=”true”]
[c]ID Q=[Qq]
[f]IFllcy4gQ2FyYm9uIGhhcyA0wqBvdXRlciAob3IgdmFsZW5jZSkgZWxlY3Ryb25zLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBCdXQgeW91IGNhbiBmaWd1cmUgaXQgb3V0IGJ5IGV4YW1pbmluZyB0aGlzIGRpYWdyYW0u
Cg==[Qq]
[q multiple_choice=”true”] In ethane, is the bond connecting the two carbons a single bond or a double bond?
[c]IHNpbm dsZQ==[Qq]
[f]IENvcnJlY3QuIFRoZSBib25kIGJldHdlZW4gdGhlIHR3byBjYXJib25zIChhbmQgYmV0d2VlbiB0aGUgY2FyYm9ucyBhbmQgdGhlIGh5ZHJvZ2VucykgaXMgYSBzaW5nbGUgYm9uZCwgaW52b2x2aW5nIGEgc2luZ2xlIHNoYXJlZCBwYWlyIG9mIGVsZWN0cm9ucy4=[Qq]
[c]IGRvdWJsZQ==[Qq]
[f]IE5vLiBXaGVuIGF0b21zIHNoYXJlIGEgc2luZ2xlIHBhaXIgb2YgZWxlY3Ryb25zLCBpdCYjODIxNztzIGEgc2luZ2xlIGJvbmQu[Qq]
[q]Because carbon has four outer electrons, it can be organized into a variety of forms. Label these forms in the table below.
| ______________ | _____________ | ____________ |
[l]branches
[f*] Good!
[fx] No. Please try again.
[l]chains
[f*] Great!
[fx] No. Please try again.
[l]rings
[f*] Excellent!
[fx] No. Please try again.
[q] In your own words, explain why carbon is the central element in living things.
[c]IFtzaG93X21lX3Bs YWNlaG9sZGVyXQ==[Qq]
[f]IENhcmJvbiBoYXMgZm91ciB2YWxlbmNlIGVsZWN0cm9ucy4gVGhlIHJlc3VsdGluZyBmbGV4aWJpbGl0eSBpbiBmb3JtaW5nIGJvbmRzIGVuYWJsZXMgY2FyYm9uLWJhc2VkIG1vbGVjdWxlcyB0byBiZSBsYXJnZSBhbmQgY29tcGxleCwgYW5kIGluY2x1ZGUgYnJhbmNoZXMsIHJpbmdzLCBhbmQgY2hhaW5zLiBUaGlzLCBpbiB0dXJuLCBjcmVhdGVzIHRoZSBraW5kIG9mIG1vbGVjdWxhciBzdHJ1Y3R1cmVzIHRoYXQgbWFrZSBsaWZlJiM4MjE3O3MgZXNzZW50aWFsIGZ1bmN0aW9ucyAoc3VjaCBhcyBlbmVyZ3kgdHJhbnNmZXIgYW5kIHN0b3JhZ2UsIGluZm9ybWF0aW9uIHN0b3JhZ2UsIHJlcHJvZHVjdGlvbiwgZXRjLikgcG9zc2libGUu[Qq]
[/qwiz]
Links
-
- Continue to the next tutorial on functional groups
- Go back to the Carbon and Functional Groups Menu