1. Monomers: A quick review
As we saw on the previous page, any account of the origin of life has to account for the abiotic synthesis of monomers.
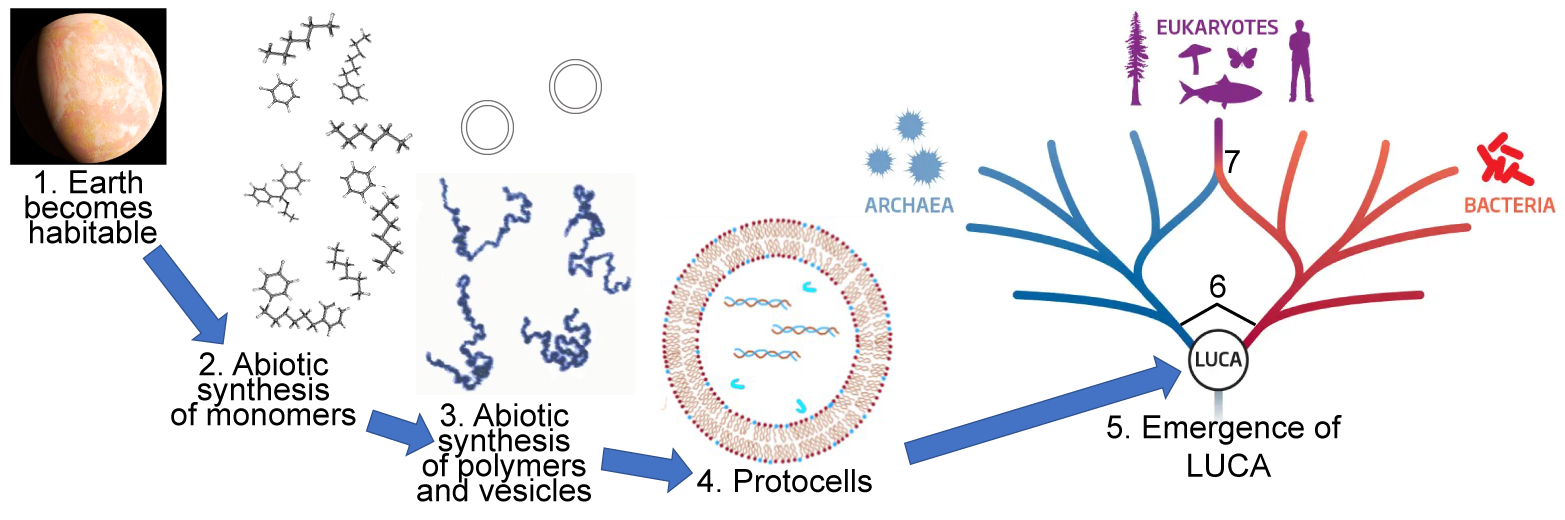
We learned a lot about monomers in Unit 1 of AP Bio. Use the quiz below to review.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Origin of Life Monomer Review (v2.0)”]
[h]Origin of life monomer review.
[i]Note the timer on your right. Move fast!
[q]An amino acid.
[textentry single_char=”true”]
[c]ID I=
[f]IEV4Y2VsbGVudC4gTnVtYmVyIDIgcmVwcmVzZW50cyBhbiBhbWlubyBhY2lkLg==[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIEFtaW5vIGFjaWRzIGhhdmUgYSBjZW50cmFsIGNhcmJvbiBhdG9tLCBmbGFua2VkIGJ5IGEgY2FyYm94eWxpYyBhY2lkIGFuZCBhbiBhbWlubyBmdW5jdGlvbmFsIGdyb3VwLg==[Qq]
[q]A monosaccharide.
[textentry single_char=”true”]
[c]ID E=
[f]IE5pY2UuIE51bWJlciAxIHJlcHJlc2VudHMgYSBtb25vc2FjY2hhcmlkZS4=[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIE1vbm9zYWNjaGFyaWRlcyBhcmUgc2ltcGxlIHN1Z2Fycy4gQSB0eXBpY2FsIGZvcm11bGEgaXMgQw==Ng==SA==MTI=Tw==[Qq]6.
[q]A nucleotide.
[textentry single_char=”true”]
[c]ID Q=
[f]IFdheSB0byBnby4gTnVtYmVyIDQgcmVwcmVzZW50cyBhIG51Y2xlb3RpZGUu[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIE51Y2xlb3RpZGVzIGhhdmUgdGhyZWUgc3VicGFydHM6IGEgbml0cm9nZW5vdXMgYmFzZSwgYSA1LWNhcmJvbiBzdWdhciwgYW5kIHRocmVlIHBob3NwaGF0ZSBncm91cHMu[Qq]
[q]A fatty acid.
[textentry single_char=”true”]
[c]ID M=
[f]IEdvb2Qgd29yay4gTnVtYmVyIDMgcmVwcmVzZW50cyBhIGZhdHR5IGFjaWQu[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIEZhdHR5IGFjaWRzIGhhdmUgYSBoeWRyb2NhcmJvbiBjaGFpbiB0aGF0IGVuZHMgKG9uIG9uZSBlbmQpIHdpdGggYSBjYXJib3h5bGljIGFjaWQu[Qq]
[q]The monomer of proteins.
[textentry single_char=”true”]
[c]ID I=
[f]IEV4Y2VsbGVudC4gQW1pbm8gYWNpZHMgKGF0IDIpIGFyZSB0aGUgbW9ub21lcnMgb2YgcHJvdGVpbnMu[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFlvdSYjODIxNztyZSBsb29raW5nIGZvciBhbiBhbWlubyBhY2lkLg==[Qq]
[q]The monomer of carbohydrates.
[textentry single_char=”true”]
[c]ID E=
[f]IE5pY2UuIE51bWJlciAxIHJlcHJlc2VudHMgYSBtb25vc2FjY2hhcmlkZSwgd2hpY2ggaXMgdGhlIG1vbm9tZXIgb2YgY2FyYm9oeWRyYXRlcy4=[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFlvdSYjODIxNztyZSBsb29raW5nIGZvciBhIG1vbm9zYWNjaGFyaWRlLg==[Qq]
[q]The monomer of nucleic acids.
[textentry single_char=”true”]
[c]ID Q=
[f]IFdheSB0byBnby4gTnVtYmVyIDQgcmVwcmVzZW50cyBhIG51Y2xlb3RpZGUsIHRoZSBtb25vbWVyIG9mIG51Y2xlaWMgYWNpZHMu[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFlvdSYjODIxNztyZSBsb29raW5nIGZvciBhIG51Y2xlb3RpZGUu[Qq]
[q]These are a component of phospholipids and triglycerides.
[textentry single_char=”true”]
[c]wq Az
[f]IEdvb2Qgd29yay4gTnVtYmVyIDMgcmVwcmVzZW50cyBhIGZhdHR5IGFjaWQsIHdoaWNoIGlzIGEgcGFydCBvZiBwaG9zcGhvbGlwaWRzIGFuZCB0cmlnbHljZXJpZGVzLg==[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFlvdSYjODIxNztyZSBsb29raW5nIGZvciBhIGZhdHR5IGFjaWQu[Qq]
[q]Associated with enzymes, membrane channels, and receptors.
[textentry single_char=”true”]
[c]ID I=
[f]IEV4Y2VsbGVudC4gQW1pbm8gYWNpZHMgKGF0IDIpIGFyZSB0aGUgbW9ub21lcnMgb2YgcHJvdGVpbnMsIHdoaWNoIGhhdmUgdGhlIGZ1bmN0aW9ucyBkZXNjcmliZWQgYWJvdmUu[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFlvdSYjODIxNztyZSBsb29raW5nIGZvciBhbiBhbWlubyBhY2lkLg==[Qq]
[q]Molecules like this are assembled during photosynthesis and are the key energy source for cellular respiration
[textentry single_char=”true”]
[c]ID E=
[f]IE5pY2UuIE51bWJlciAxIHJlcHJlc2VudHMgYSBtb25vc2FjY2hhcmlkZSwgd2hpY2ggaGFzIHRoZSBwcm9wZXJ0aWVzIGRlc2NyaWJlZCBhYm92ZS4=[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFlvdSYjODIxNztyZSBsb29raW5nIGZvciBhIG1vbm9zYWNjaGFyaWRlLg==[Qq]
[q]Associated with information storage; also with ATP.
[textentry single_char=”true”]
[c]ID Q=
[f]IFdheSB0byBnby4gTnVtYmVyIDQgcmVwcmVzZW50cyBhIG51Y2xlb3RpZGUsIHdoaWNoIGhhcyB0aGUgcHJvcGVydGllcyBkZXNjcmliZWQgYWJvdmUu[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFlvdSYjODIxNztyZSBsb29raW5nIGZvciBhIG51Y2xlb3RpZGUu[Qq]
[q]These are key components of the molecules that make up cell membranes.
[textentry single_char=”true”]
[c]ID M=
[f]IEdvb2Qgd29yay4gTnVtYmVyIDMgcmVwcmVzZW50cyBhIGZhdHR5IGFjaWQsIHdoaWNoIGlzIGEgcGFydCBvZiBwaG9zcGhvbGlwaWRzLCB3aGljaCBtYWtlIHVwIGNlbGwgbWVtYnJhbmVzLg==[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFlvdSYjODIxNztyZSBsb29raW5nIGZvciBhIGZhdHR5IGFjaWQu[Qq]
[x]
[restart]
[/qwiz]
2. Abiotic Formation of Monomers: The Oparin-Haldane Hypothesis
Here are a few general points about monomers as they relate to the origin of life.
- Monomers are relatively small and simple (compared to polymers). But, with up to a few dozen atoms, they’re more complex than molecules like water and carbon dioxide, which form abiotically and are found throughout our solar system.
- They’re all chemically reduced, high-energy molecules. They’re full of hydrogen atoms, which have highly energetic electrons.
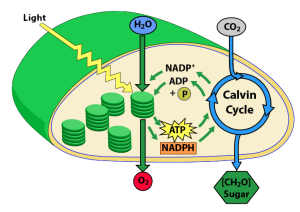
On Earth today (and for the last 3.5 billion years), monomers are made biotically. Plants and other autotrophs (self-feeding organisms) make their monomers from carbon dioxide and water, which are combined during photosynthesis into the three-carbon sugar G3P (glyceraldehyde-3-phosphate), which is then synthesized into glucose (and everything else). This reaction is endergonic: it’s only possible because plants and other photoautotrophs can harness light energy from the sun.
So, in terms of the origin of life, what we have to explain is how, in the absence of life, monomers could come about.
In the 1920s, two scientists, Alexander Oparin and J.B.S. Haldane, independently hypothesized a way that this could happen. Under the conditions that were then thought to have prevailed on the early Earth, they thought that monomers could spontaneously arise. These monomers would accumulate in the early oceans, forming what’s called a primordial or prebiotic soup. This soup would subsequently serve as the source of monomers for the creation of polymers.

Oparin and Haldane knew that early Earth’s atmosphere would have been oxygen-free because molecular oxygen (O2) results from photosynthesis. They proposed, instead, that the early atmosphere would be chemically reducing, containing gases such as hydrogen (H2) and methane (CH4)
In today’s oxygen-rich atmosphere, the spontaneous direction of chemical reactions is to break down complex molecules into simpler ones. That’s why metals rust and wood burns: both rusting and burning are oxidation reactions. By contrast, Oparin and Haldane thought that in a reducing atmosphere, the opposite would occur. More complex molecules would form from simpler ones. And that, thought Oparin and Haldane, would be how the first monomers would arise. Their idea came to be known as the Oparin-Haldane hypothesis.
3. Testing the Oparin-Haldane Hypothesis: the Miller-Urey Experiment.

In 1953, the Oparin-Haldane hypothesis was tested by Stanley Miller, who at that time was a 23-year-old graduate student at the University of Chicago. Miller was working with Harold Urey, a winner of the Nobel Prize for chemistry. Their experiment is now known as the Miller-Urey Experiment. For an atmospheric mix, Miller chose to use the gases that surround the outer, gas giant planets (like Jupiter and Saturn). These planets have highly reducing atmosphere that consists of methane (CH4), molecular hydrogen (H2), water, and ammonia (NH3).
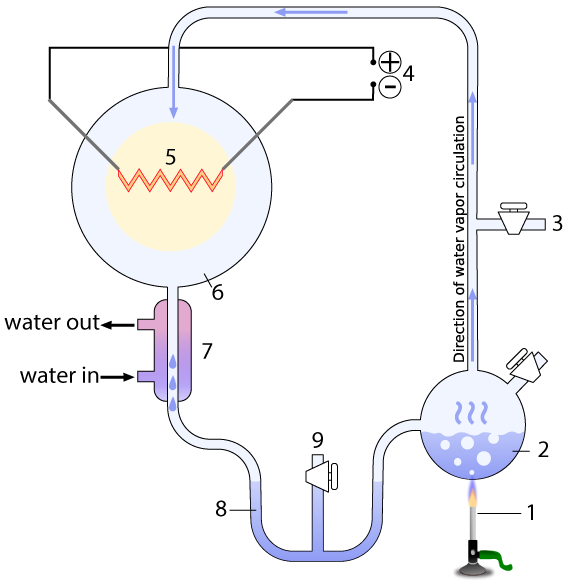
The entire apparatus was a closed, sterile system, with various valves that could be used to vacuum out all air from the system (3), and sample what the system was producing without contaminating it (9). The system contained a water-filled chamber that simulated the ancient ocean (2). A heat source (1) heated the “ocean,” creating water vapor that would rise into a chamber that represented the primitive atmosphere (6) with methane (CH4), ammonia (NH3), molecular hydrogen (H2), and water vapor (H2O)
The apparatus also contained electrodes (4) which could produce sparks (5), simulating lightning. A condenser (7) cooled the circulating gas. The cooled gas condensed as a liquid in a trap (8), which allowed Miller to see what his apparatus was brewing up.
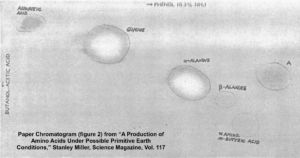
After five days, Miller stopped the experiment and sampled the contents. To detect what had formed within his apparatus, Miller used a technique called paper chromatography. This involves using solvents that rise up a paper column, carrying with them dissolved substances, which will rise a known amount in a specific period of time.
You can see the results in the photograph Miller published in his May 1953 paper, which was entitled “A Production of Amino Acids under Possible Primitive Earth Conditions” (click the previous link to read: it’s only two pages). The specific amino acids Miller detected were aspartic acid, glycine, alpha and beta-alanine, and α-aminobutyric acid. Abiotic synthesis of monomers seemed to have been confirmed, and Miller’s experiment was widely reported in the press.
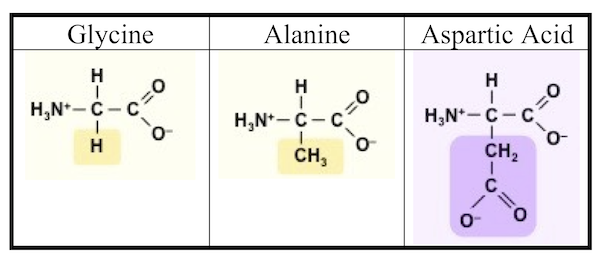
It turns out that Miller might have been more successful than he knew. In 2007, the year Miller died, preserved results of his earliest experiments (his work from 1953, and in subsequent years) were reanalyzed. It was found that Miller’s spark-chamber experiments had yielded not five amino acids, but thirty-one, along with 12 dipeptides (two amino acids linked together by a peptide bond). You can read this analysis of Miller’s results in “One of the Foremost Experiments of the Twentieth Century.”
On the other hand, Miller’s work has been criticized for using an atmospheric mix that was too reduced, and thus too conducive to the formation of organic compounds. The Earth’s ancient atmosphere probably didn’t have significant amounts of molecular hydrogen or methane. But Miller’s work inspired many other experiments, which have used different mixes of atmospheric gases, energy sources, and minerals (such as iron), and have resulted in the production of the nitrogenous bases found in RNA and DNA (though not complete nucleotides) and a wide array of other amino acids.

Can monomers form naturally under abiotic conditions? In the years since Miller’s first experiment, amino acids and nucleotide bases have been found inside meteorites (which form out in space) and have also been detected in comets. This includes the Murchison meteorite, which landed in Australia in 1969 and was subject to chemical analysis.
What’s the takeaway? It seems probable that within a few hundred million years of the Earth’s cooling, some of the monomers that would make up the polymers within the first living organisms might have spontaneously formed.
We’ll look at scenarios for polymer formation in the next tutorial. But first, let’s consolidate our understanding of the Oparin-Haldane hypothesis and the Miller-Urey experiment.
4. The Miller-Urey Experiment: Checking Understanding
[qwiz random = “true” qrecord_id=”sciencemusicvideosMeister1961-The Miller-Urey Experiment (v2.0)”]
[h]The Miller-Urey Experiment
[i]
[q] In the Miller-Urey experiment, which number represents the part that simulated the ancient oceans?
[textentry single_char=”true”]
[c]ID I=
[f]IEV4Y2VsbGVudC4gTnVtYmVyIDIgcmVwcmVzZW50cyB0aGUgd2F0ZXIgaW4gdGhlIGFuY2llbnQgb2NlYW5zLg==[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFdoaWNoIHBhcnQgbG9va3MgbGlrZSBpdCB3b3VsZCBjb250YWluIHdhdGVyPw==[Qq]
[q] In the Miller-Urey experiment, which number represents simulated lightning in the ancient atmosphere?
[textentry single_char=”true”]
[c]ID U=
[f]IE5pY2Ugam9iLiBOdW1iZXIgNSByZXByZXNlbnRzIGxpZ2h0bmluZyBpbiB0aGUgYW5jaWVudCBhdG1vc3BoZXJlLg==[Qq]
[c]ID Q=[Qq]
[f]IE9rLiBUaGF0IHdhc24mIzgyMTc7dCBleGFjdGx5IHdoYXQgdGhlIHF1ZXN0aW9uIHdhcyBhc2tpbmcsIGJ1dCBpdCYjODIxNztzIGNsb3NlIGVub3VnaDogJiM4MjIwOzQmIzgyMjE7IHJlcHJlc2VudHMgZWxlY3Ryb2RlcyB0aGF0IGNyZWF0ZWQgdGhlIHNwYXJrcyB0aGF0IHNpbXVsYXRlZCBsaWdodG5pbmcgaW4gdGhlIGFuY2llbnQgYXRtb3NwaGVyZS4=[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFdoaWNoIHBhcnQgbG9va3MgbGlrZSBpdCB3b3VsZCByZXByZXNlbnQgYW4gZWxlY3RyaWNhbCBzcGFyaz8=[Qq]
[q] In the Miller-Urey experiment, which number represents a part that simulated heat from volcanoes?
[textentry single_char=”true”]
[c]ID E=
[f]IEdvb2Qgd29yay4gTnVtYmVyIDEgcmVwcmVzZW50cyBoZWF0IGZyb20gYW5jaWVudCB2b2xjYW5vZXMu[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFdoaWNoIHBhcnQgbG9va3MgbGlrZSBpdCB3b3VsZCBwcm9kdWNlIGhlYXQ/[Qq]
[q] In the diagram below of the Miller-Urey experiment, the electrodes that produce the lighting are represented by which number?
[textentry single_char=”true”]
[c]ID Q=
[f]V2F5IHRvIGdvLiBOdW1iZXIgNCByZXByZXNlbnRzIHRoZSBzcGFyay1wcm9kdWNpbmcgZWxlY3Ryb2Rlcy4=[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIEVsZWN0cmljaXR5IGhhcyBwb3NpdGl2ZSBhbmQgbmVnYXRpdmUgY2hhcmdlcy4gV2hlcmUgZG8geW91IHNlZSBzeW1ib2xzIHRoYXQgd291bGQgcmVwcmVzZW50IHBsdXMgYW5kIG1pbnVzIGNoYXJnZXM/[Qq]
[q] In the diagram below of the Miller-Urey experiment, the chamber that contains the gases in the ancient atmosphere would be found at which number?
[textentry single_char=”true”]
[c]Ng ==
[f]Q29ycmVjdC4gTnVtYmVyIDYgcmVwcmVzZW50cyB0aGUgYW5jaWVudCBhdG1vc3BoZXJlLg==[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFRoZSBsaWdodGluZyBvY2N1cnMgd2l0aGluIHRoZSBhdG1vc3BoZXJlLg==[Qq]
[q] In the diagram below of the Miller-Urey experiment, the condenser that cooled the gases in the atmosphere, causing whatever was in the atmosphere to precipitate into the trap, is found at which number?
[textentry single_char=”true”]
[c]Nw ==
[f]TmljZS4gTnVtYmVyIDcgcmVwcmVzZW50cyB0aGUgY29uZGVuc2VyLg==[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIENvbmRlbnNlcnMgY2lyY3VsYXRlIHdhdGVyIHRoYXQgY2F1c2VzIHZhcG9ycyB0byBjb25kZW5zZS4gRmluZCB3aGVyZSB3YXRlciBpcyBjaXJjdWxhdGluZyBpbiBhbmQgb3V0Lg==[Qq]
[q] In the diagram below of the Miller-Urey experiment, where would amino acids and other organic compounds have been collected?
[textentry single_char=”true”]
[c]OA ==
[f]TmljZS4gTnVtYmVyIDggcmVwcmVzZW50cyB0aGUgdHJhcCwgYW5kIHRoYXQmIzgyMTc7cyB3aGVyZSBvcmdhbmljIGNvbXBvdW5kcyBsaWtlIGFtaW5vIGFjaWRzIHdvdWxkIHByZWNpcGl0YXRlIGZvciBjb2xsZWN0aW9uLg==[Qq]
[c]ICo=[Qq]
[f]Tm8uIEhlcmUmIzgyMTc7cyBhIGhpbnQuIFRoZSB0cmFwIGlzIGJlbmVhdGggdGhlIGNvbmRlbnNlci4=[Qq]
[q]In the Miller-Urey experiment, the mixture of gases used in the experiment differed from the gases in the current atmosphere in many ways. For one thing, [hangman] (a gas produced by photosynthesis) was lacking.
[c]b3h5Z2Vu[Qq]
[q]Because of the presence of oxygen, today’s atmosphere is oxidizing. In the Oparin-Haldane hypothesis, the atmosphere is thought to be [hangman].
[c]cmVkdWNpbmc=[Qq]
[q]The Miller-Urey experiment was designed to prove that [hangman] synthesis of monomers might have been possible on the early Earth
[c]YWJpb3RpYw==[Qq]
[q]According to the Oparin-Haldane hypothesis, the abiotic synthesis of monomers would result in the creation of a [hangman] soup, in which life would subsequently emerge.
[c]cHJpbW9yZGlhbA==[Qq]
[x][restart]
[/qwiz]
5. Three reasons why the abiotic production of polymers is difficult
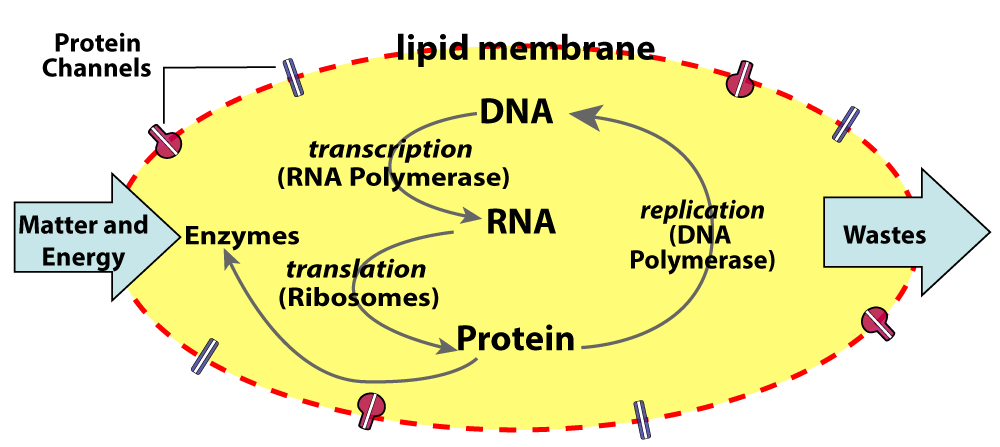
After the abiotic production of monomers, the production of polymers is the next step in chemical evolution. In any origin-of-life scenario, this might be the hardest step to explain. Here are three reasons why.
Reason 1: You need Polymers to Make Polymers
The first problem is like the “which came first, the chicken or the egg?” riddle. In living systems today, making proteins and nucleic acids (RNA and DNA) requires proteins and nucleic acids. For example, cells use ribosomes (made of RNA and protein) and messenger RNA (made of RNA) to synthesize proteins. To synthesize RNA, cells use the enzyme RNA polymerase (a protein) which reads a DNA template. DNA replication requires a whole team of enzymes (all proteins). So, how, during the emergence of life, could nucleic acids and proteins form in the absence of already existing nucleic acids and proteins?
Reason 2: The presence of water works against polymer formation
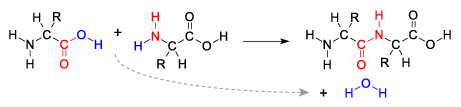
The second problem is referred to as the water paradox. The paradox is that life is based on water, but the presence of water makes abiotic polymer formation difficult. Here’s why.
Monomers that are dissolved in water only form polymers in the presence of enzymes. That’s because monomers form polymers through dehydration synthesis reactions. In these reactions, water molecules are formed as enzymes remove a water molecule from the monomers by combining a hydrogen atom with a hydroxyl group. At the same time, the enzyme catalyzes the bond between the two monomers.
In terms of the origin of life, the problem is that the presence of water works against dehydration synthesis, and promotes hydrolysis. Hydrolysis is the opposite of dehydration synthesis: it’s when enzymes split polymers apart by jamming the parts of a water molecule between the monomers in a polymer. Since life today is based on water and since life probably evolved in water, it’s hard to see how, in the absence of enzymes, polymers could spontaneously form and accumulate.
Reason 3: Polymer formation is endergonic. What energy source powered the process?
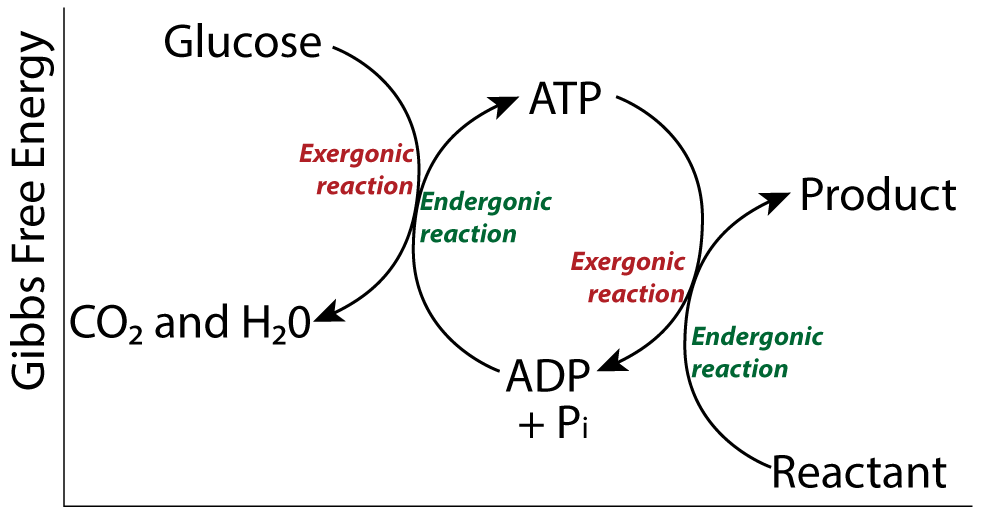
In addition to enzymes, creating polymers from monomers requires energy. Dehydration synthesis is an endergonic reaction. It reduces entropy. In living cells, the energy for polymer creation comes from energy coupling involving ATP. But with no ATP present, what moved these reactions forward?
6. Scenarios for Abiotic Polymer formation
Here are a few scenarios explaining how chemical evolution could have led to the synthesis of polymers from monomers on early Earth.
6a. Inorganic catalysts could have taken the role of enzymes

In undersea hydrothermal vents, which are widely present throughout the oceans, various reactions result in the accumulation of metal catalysts. While these catalysts are not as effective or as specific as enzymes at speeding up chemical reactions, they have been shown to catalyze polymerization.
For example, Gunter Wachtershauser and Claudia Huber, working in Germany, have shown that peptides (small chains of amino acids) can form under vent-like conditions in the presence of nickel-iron sulfide catalysts.
Wachtershauser has gone on to propose an entire paradigm for chemical evolution that’s called the Iron-Sulfur world hypothesis, which also explains the origin of monomers. You can read about it on Wikipedia.
Clay minerals can also act as catalysts. These minerals are present all over the Earth’s surface (both land and sea). A variety of experiments by origin-of-life researchers have demonstrated that clay minerals can catalyze the polymerization of amino acids into polypeptides and RNA nucleotides into RNA polymers. For additional information, try this article or this one (but note that the biochemistry in both of them is pretty advanced).
6b. Rocky surfaces, heat, and drying can assemble monomers into polymers
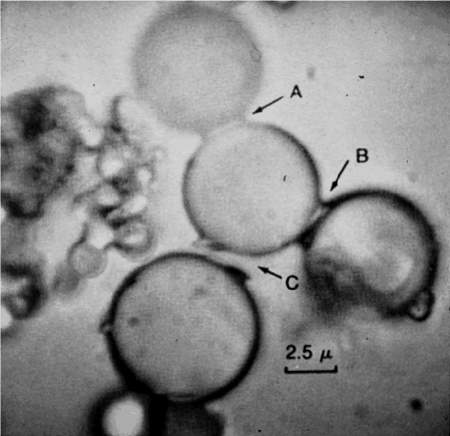
University of Miami Professor Sydney Fox (1912 – 1998) showed that exposing amino acids to dry heat could cause them to polymerize. Fox promoted a scenario where amino acids that formed in the primordial soup would wash up on rocky shorelines. There, exposed to heat from the sun, they could form small polypeptides that Fox called proteinoids. Dissolving proteinoids in water led them to form structures that Fox called proteinoid microspheres, which Fox claimed to have properties that were similar to cells.
Fox’s work inspired other scientists who are creating scenarios and laboratory simulations for the abiotic synthesis of polymers through heating and drying out monomers.
It’s even been proposed that the best location for this process was not on Earth, but on Mars. Mars is now a desert planet, but once had abundant water. The idea is that processes of heating and drying on the Martian surface led to the chemical evolution of monomers and polymers, which led to the first bacteria-like cells. Asteroid impacts billions of years ago would have blasted bacteria-infested Martian rocks into space. The bacteria survived the trip through space to Earth, where they seeded the Earth with life. That means that both you and I (according to this scenario) have Martian ancestors.
You can read about Fox’s work on Wikipedia. Click the following link for a review of the water paradox and, possible solutions. For more about the Martian origins hypothesis, see this article in Smithsonian Magazine, this much more detailed journal article, or read pages 58 to 60 in A New History of Life, by Peter Ward and Joe Hirschvink).
7. Polymer Formation: Checking Understanding
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Polymer Formation in the Origin of Life (v2.0)”]
[h]Polymer Formation in the Origin of Life
[i]
[q] A problem associated with any account of abiotic polymer formation is that in living systems today, monomers form into polymers through[hangman] synthesis reactions, which are catalyzed by [hangman]
[c]IGRlaHlkcmF0aW9u[Qq]
[c]IGVuenltZXM=[Qq]
[q] In today’s living systems, amino acids are polymerized through the action of a two-unit molecular machine called a [hangman]. Any origin of life scenario needs to be able to explain how with these being absent, [hangman], the polymers of amino acids, could still form.
[c]IHJpYm9zb21l[Qq]
[c]IHByb3RlaW5z[Qq]
[q]The origin-of-life water paradox runs like this: life is based on water, but the presence of water promotes [hangman], which breaks polymers into [hangman]. Living things solve this process today through the action of [hangman], which weren’t present when life was first emerging.
[c]aHlkcm9seXNpcw==[Qq]
[c]bW9ub21lcnM=[Qq]
[c]ZW56eW1lcw==[Qq]
[q]Creating polymers from monomers is a(n) [hangman] reaction. In cells today, polymer creation is driven forward by coupling with the breakdown of [hangman] to [hangman]. So another origin-of-life question is how to form polymers from monomers in the absence of today’s energy-coupling system.
[c]ZW5kZXJnb25pYw==[Qq]
[c]QVRQ[Qq]
[c]QURQ[Qq]
[q]Some models for explaining polymer synthesis promote the idea that metal-based, inorganic [hangman] could have played the same role currently played by [hangman] in getting monomers to combine into polymers
[c]Y2F0YWx5c3Rz[Qq]
[c]ZW56eW1lcw==[Qq]
[q]Research Sydney Fox demonstrated that drying out solutions of [hangman] acids could get them to bond together, forming small polypeptide chains that Fox called [hangman]
[c]YW1pbm8=[Qq]
[c]cHJvdGVpbm9pZHM=[Qq]
[q]Because dry environments are more conducive to the polymerization of monomers to polymers, some origin-of-life theorists have proposed that life might have emerged on the planet [hangman]. Asteroid impacts would have blasted [hangman]-infested rocks off the surface of that planet. When these rocks landed on Earth, they seeded the Earth with life.
[c]TWFycw==[Qq]
[c]YmFjdGVyaWE=[Qq]
[x][restart]
[/qwiz]
8. What’s Next?
Proceed to Topic 7.12, Part 3: the RNA World and the Formation of Cells (the next tutorial in Topic 7.12, The Origin of Life).
