1. Watch this Video
2. Study this Summary
Protein functions
Proteins are life’s molecule of action. Here are some of the key functions that proteins play in living things:
- Motion (as in the proteins actin and myosin, which interact to create muscle tissue);
- Controlling chemical reactions as enzymes;
- Structure (as in the flexible protein collagen or the more fibrous keratin, which makes hair, feathers, and nails;
- Transport (as in hemoglobin, which carries oxygen)
- Energy storage (albumin)
- Transmitting signals (as in protein hormones like insulin or protein neurotransmitters like serotonin).
Amino acids are the monomers of proteins
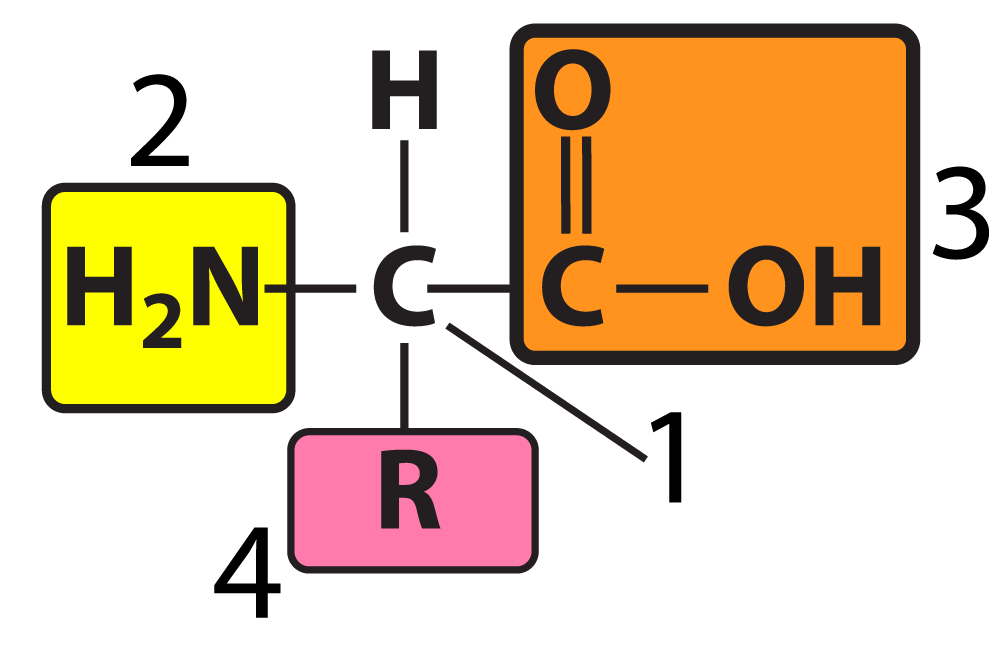
- Components:
- Central carbon atom.
- Amine group (basic).
- Carboxyl group (acidic).
- Hydrogen atom.
- Variable R group (side chain): Can be polar, non-polar, acidic, or basic.
- All life uses the same 20 amino acids.
Levels of Protein Structure
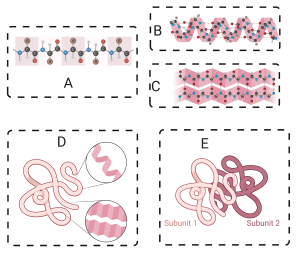
- Primary Structure:
- Linear sequence of amino acids linked by peptide bonds.
- Sequence is genetically determined.
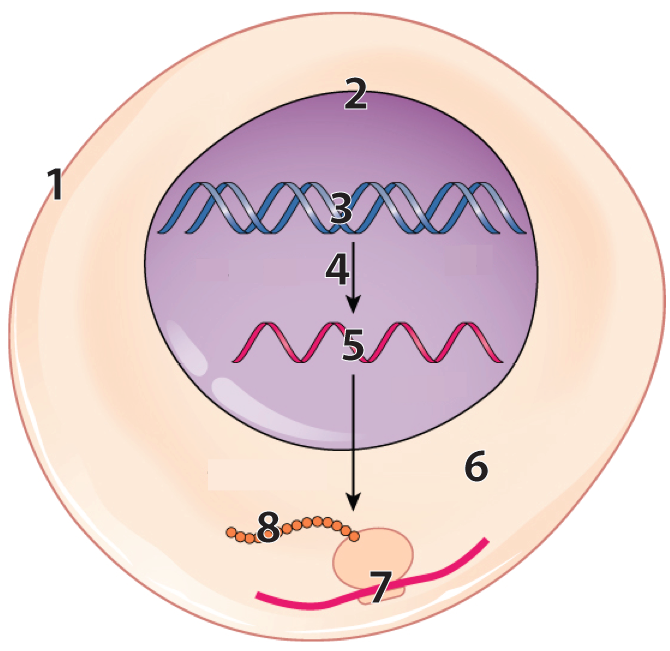
- Built by ribosomes, not enzymes. In the diagram below, 7 shows ribosome “reading” mRNA (at 5), and translating it into a polypeptide (at 8).
- Forms the polypeptide backbone (alternating carbon and nitrogen atoms).
- Secondary Structure:
- Stabilized by hydrogen bonds between amine and carbonyl groups in the polypeptide backbone.
- Key shapes:
- Alpha helix: Corkscrew shape stabilized by hydrogen bonds.
- Beta-pleated sheet: Parallel or antiparallel strands stabilized by hydrogen bonds.
- Tertiary Structure:
- Interactions between R groups:
- Hydrogen bonds.
- Ionic bonds.
- Covalent bonds (e.g., disulfide bonds between sulfhydryl groups).
- Hydrophobic clustering: Non-polar side chains cluster to avoid water.
- Example: Myoglobin, an oxygen-storing protein in muscles.
- Interactions between R groups:
- Quaternary Structure:
- Involves interactions between multiple folded polypeptide chains.
- Bonds include hydrogen bonds, ionic bonds, and hydrophobic interactions.
- Examples:
- Hemoglobin: Four polypeptide chains transport oxygen in red blood cells.
- SARS-CoV-2 spike protein: Multiple polypeptide chains form the structure.
Illustrative Example: Hemoglobin and Sickle Cell Disease
- Hemoglobin Structure:
- Quaternary protein composed of four polypeptide chains.
- Cause:
- Mutation substituting valine (non-polar) for glutamic acid (acidic) in hemoglobin.
- Results in hydrophobic interactions between hemoglobin molecules in deoxygenated blood.
- Effects:
- Formation of fibers within red blood cells, causing a spiked shape.
- Mutant cells clump in small arteries, leading to pain crises, tissue damage, and other symptoms.
3. Master these Flashcards
[qdeck qrecord_id=”sciencemusicvideosMeister1961-Proteins Flashcards, APBVP”]
[h]Proteins
[i]
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” dataset_id=”AP_Bio_Flashcards_2022|1f1607a7aa110″ question_number=”30″ topic=”1.5-6.Proteins_and_Nucleic_Acids”] Name the monomer of proteins, and describe that monomer’s structure.
[a] Proteins are polymers of amino acids. Amino acids are built around a central carbon, attached to an amine group (2), a carboxyl group (3), a hydrogen atom, and a variable R group (4). The R group also called a “side chain” can be polar, non-polar, acidic, or basic. Interactions between amino acids (covered in another card) determine the protein’s three-dimensional shape.
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” dataset_id=”AP_Bio_Flashcards_2022|1f0bfd3462d10″ question_number=”31″ topic=”1.5-6.Proteins_and_Nucleic_Acids”] Describe the biological importance of proteins. See if you can list 5 key functions of proteins. Here’s a hint: each functions starts with one of the following letters: MESTES
[a] Proteins functions include
- Motion (as in the proteins actin and myosin, which interact to create muscle tissue);
- Controlling chemical reactions as enzymes;
- Structure (as in the flexible protein collagen or the more fibrous keratin, which makes hair, feathers, and nails;
- Transport (as in hemoglobin, which carries oxygen)
- Energy storage (albumin)
- Transmitting signals (as in protein hormones like insulin or protein neurotransmitters like serotonin).
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” question_number=”32″ topic=”1.5-6.Proteins_and_Nucleic_Acids” dataset_id=”AP_Bio_Flashcards_2022|6535dac8b8285″] Describe primary protein structure.
[a] The first level of structure is primary structure: it consists of the sequence of amino acids in a polypeptide chain. This sequence is genetically determined and emerges as ribosomes translate messenger RNA into polypeptide chains, with each amino acid’s position and identity spelled out by codons (3 base sequences) in mRNA.
In the diagram below, each sphere represents one of the linked amino acids making up a protein’s primary structure.
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” question_number=”33″ topic=”1.5-6.Proteins_and_Nucleic_Acids” dataset_id=”AP_Bio_Flashcards_2022|65282d02dca85″] Describe secondary protein structure.
[a] Secondary protein structure involves interactions between carbonyl groups and amino groups in the polypeptide backbone. These interactions can cause a polypeptide to twist into a coiled alpha helix (left), or form a regularly folded structure called a pleated sheet (right).
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” question_number=”34″ topic=”1.5-6.Proteins_and_Nucleic_Acids” dataset_id=”AP_Bio_Flashcards_2022|11a98dedfa14a9″] Describe tertiary protein structure.
[a] Interactions between amino acid side chains (also called R-groups) result in a tertiary structure. These interactions involve hydrogen bonds (2), ionic bonds (5), covalent bonds (3), or hydrophobic clustering (4). The result is a complex, three-dimensional shape.
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” question_number=”35″ topic=”1.5-6.Proteins_and_Nucleic_Acids” dataset_id=”AP_Bio_Flashcards_2022|11a8a0713d3ca9″] Describe quaternary protein structure.
[a] Multiple polypeptides can interact to form a quaternary structure, which is found in proteins such as hemoglobin (shown below), which consists of four, interconnected, polypeptides. The bonds that stabilize a quaternary structure include hydrogen bonds, ionic bonds, and hydrophobic interactions.
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” dataset_id=”AP_Bio_Flashcards_2022|1ee1a49c20110″ question_number=”37″ topic=”1.5-6.Proteins_and_Nucleic_Acids”] Describe the structure and function of hemoglobin, and explain the molecular cause of sickle cell disease.
[a] Hemoglobin transports oxygen in red blood cells. It’s a globular protein made of four polypeptide chains. Two of these are identical alpha chains, and two are identical beta chains. The beta chain consists of 146 amino acids.
People with sickle cell disease are homozygous for a recessive mutation in which the amino acid valine (with a nonpolar side chain) substitutes for glutamic acid (with an acidic side chain). Consequently, when blood becomes deoxygenated, the mutated hemoglobin molecules form hydrophobic bonds with one another, causing them to aggregate into fibers (as shown in B). This reduces hemoglobin’s capacity to carry oxygen and deforms the shape of red blood cells, which become elongated and spiked. This causes these cells to become trapped in capillaries, blocking blood flow and causing pain and tissue damage.
[x]
[restart]
[/qdeck]
4. Tackle these Quizzes
4.1. Amino Acids, Peptide Bonds, and Polypeptides
[h]Amino Acids, Peptide Bonds, and Polypeptides
[i]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem_7_amino acids and peptide bonds|e9225fb4e63fa” question_number=”3″] The monomers of proteins are
[c]IGFtaW5vIG FjaWRzLg==[Qq]
[f]IEV4Y2VsbGVudC4gQW1pbm8gYWNpZHMgYXJlIHRoZSBtb25vbWVycyBvZiBwcm90ZWlucy4=[Qq]
[c]IGZhdHR5IGFjaWRzLg==[Qq]
[f]IE5vLiBGYXR0eSBhY2lkcyBhcmUgYSBrZXkgYnVpbGRpbmcgYmxvY2sgb2YgbWFueSBsaXBpZHMu[Qq]
[c]IG1vbm9zYWNjaGFyaWRlcy4=[Qq]
[f]IE5vLiBNb25vc2FjY2hhcmlkZXMgYXJlIHRoZSBtb25vbWVycyBvZiBjYXJib2h5ZHJhdGVz[Qq]
[c]IG51Y2xlb3RpZGVzLg==[Qq]
[f]IE5vLiBOdWNsZW90aWRlcyBhcmUgdGhlIG1vbm9tZXJzIG9mIG51Y2xlaWMgYWNpZHMgKGxpa2UgRE5BIGFuZCBSTkEp[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem_7_amino acids and peptide bonds|e91e4ca0173fa” question_number=”4″] In the diagram below, which number indicates the central carbon atom?
[c]ID E=[Qq]
[c]IDI=[Qq]
[c]IDM=[Qq]
[c]IDQ=[Qq]
[f]IEV4Y2VsbGVudCEgJiM4MjIwOzEmIzgyMjE7IGlzIHRoZSBjZW50cmFsIGNhcmJvbg==[Qq]
[f]IE5vLiAmIzgyMjA7MiYjODIyMTsgaXMgYW4gYW1pbm8gZ3JvdXAu[Qq]
[f]IE5vLiAmIzgyMjA7MyYjODIyMTsgaXMgdGhlIGNhcmJveHlsaWMgYWNpZA==[Qq]
[f]IE5vLiAmIzgyMjA7NCYjODIyMTsgaXMgdGhlICYjODIyMDtSJiM4MjIxOyBncm91cC4gSXQmIzgyMTc7cyB0aGUgcGFydCB0aGF0IHZhcmllcyBmcm9tIG9uZSBhbWlubyBhY2lkIHRvIHRoZSBuZXh0Lg==[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem_7_amino acids and peptide bonds|e91a144a89ffa” question_number=”5″] In the diagram below, which number indicates the amino group?
[c]IDE=[Qq]
[c]ID I=[Qq]
[c]IDM=[Qq]
[c]IDQ=[Qq]
[f]IE5vICYjODIyMDsxJiM4MjIxOyBpcyB0aGUgY2VudHJhbCBjYXJib24=[Qq]
[f]IEdyZWF0IGpvYi4gJiM4MjIwOzImIzgyMjE7IGlzIGFuIGFtaW5vIGdyb3VwLg==[Qq]
[f]IE5vLiAmIzgyMjA7MyYjODIyMTsgaXMgdGhlIGNhcmJveHlsaWMgYWNpZA==[Qq]
[f]IE5vLiAmIzgyMjA7NCYjODIyMTsgaXMgdGhlICYjODIyMDtSIGdyb3VwJiM4MjIxOyBvciAmIzgyMjA7c2lkZSBjaGFpbi4mIzgyMjE7[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem_7_amino acids and peptide bonds|e9160135baffa” question_number=”6″] In the diagram below, which number indicates the carboxylic acid?
[c]IDE=[Qq]
[c]IDI=[Qq]
[c]ID M=[Qq]
[c]IDQ=[Qq]
[f]IE5vLiAmIzgyMjA7MSYjODIyMTsgaXMgdGhlIGNlbnRyYWwgY2FyYm9u[Qq]
[f]IE5vLiAmIzgyMjA7MiYjODIyMTsgaXMgYW4gYW1pbm8gZ3JvdXAu[Qq]
[f]IFdheSB0byBnbyEgJiM4MjIwOzMmIzgyMjE7IGlzIHRoZSBjYXJib3h5bGljIGFjaWQ=[Qq]
[f]IE5vLiAmIzgyMjA7NCYjODIyMTsgaXMgdGhlICYjODIyMDtSIGdyb3VwJiM4MjIxOyBvciAmIzgyMjA7c2lkZSBjaGFpbi4mIzgyMjE7[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem_7_amino acids and peptide bonds|e911c8e02dbfa” question_number=”7″] In the diagram below, which number indicates the part that varies from one amino acid to the next?
[c]IDE=[Qq]
[c]IDI=[Qq]
[c]IDM=[Qq]
[c]ID Q=[Qq]
[f]IE5vLiAmIzgyMjA7MSYjODIyMTsgaXMgdGhlIGNlbnRyYWwgY2FyYm9uLCB3aGljaCBpcyB0aGUgc2FtZSBpbiBldmVyeSBhbWlubyBhY2lkLg==[Qq]
[f]IE5vLiAmIzgyMjA7MiYjODIyMTsgaXMgYW4gYW1pbm8gZ3JvdXAsIHdoaWNoIGlzIHRoZSBzYW1lIGluIGV2ZXJ5IGFtaW5vIGFjaWQu[Qq]
[f]IE5vICYjODIyMDszJiM4MjIxOyBpcyB0aGUgY2FyYm94eWxpYyBhY2lkLCB3aGljaCBpcyB0aGUgc2FtZSBpbiBldmVyeSBhbWlubyBhY2lkLg==[Qq]
[f]IFllcyEgJiM4MjIwOzQmIzgyMjE7IGlzIHRoZSAmIzgyMjA7UiBncm91cCwmIzgyMjE7IHRoZSBwYXJ0IHRoYXQgY2FuIHZhcnkgZnJvbSBvbmUgYW1pbm8gYWNpZCB0byB0aGUgbmV4dC4=[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_7_amino acids and peptide bonds|e90d908aa07fa” question_number=”8″] In the diagram below, which number indicates a peptide bond?
[textentry single_char=”true”]
[c]ID Q=[Qq]
[f]IEV4Y2VsbGVudCEgJiM4MjIwOzQmIzgyMjE7IGlzIHBvaW50aW5nIHRvIHRoZSBwZXB0aWRlIGJvbmQu[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBGaW5kIHRoZSBib25kIHRoYXQgY29ubmVjdHMgdGhlIGFtaW5vIGFjaWRzIGF0ICYjODIyMDsxJiM4MjIxOyBhbmQgJiM4MjIwOzMmIzgyMjE7ICh0aGUgcmVhY3RhbnRzKSBpbiB0aGUgZGlwZXB0aWRlIGF0ICYjODIyMDs1JiM4MjIxOyAodGhlIHByb2R1Y3QpLiBSZW1lbWJlciB0aGF0IHBlcHRpZGUgYm9uZHMgaW52b2x2ZSB0aGUgcmVtb3ZhbCBvZiBhbiAmIzgyMjA7SCYjODIyMTsgYW5kIGFuICYjODIyMDstT0gmIzgyMjE7IHRvIGZvcm0gYSB3YXRlciBtb2xlY3VsZS4=[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_7_amino acids and peptide bonds|e900c2493a7fa” question_number=”11″] In the diagram below, which letter shows a bond connecting a central carbon to an amino group?
[textentry single_char=”true”]
[c]IG E=[Qq]
[f]IEV4Y2VsbGVudCEgVGhlIGxldHRlciAmIzgyMjA7YSYjODIyMTsgaXMgcG9pbnRpbmcgdG8gYSBib25kIGJldHdlZW4gdGhlIGNlbnRyYWwgY2FyYm9uIG9mIHRoZSAxc3QgYW1pbm8gYWNpZCBpbiB0aGlzIHBvbHlwZXB0aWRlIGNoYWluLCBhbmQgYW4gYW1pbm8gZ3JvdXAu[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgYm9uZCByZWZlcnJlZCB0byBpbiB0aGlzIHF1ZXN0aW9uIGlzIGluIHRoZSBmaXJzdCBhbWlubyBhY2lkIGluIHRoaXMgcG9seXBlcHRpZGUgY2hhaW4u[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_7_amino acids and peptide bonds|e8e41776d33fa” question_number=”17″] In the diagram below, which letter indicates a non-polar R group?
[textentry single_char=”true”]
[c]IG k=[Qq]
[f]IEF3ZXNvbWUhIFRoZSBsZXR0ZXIgJiM4MjIwO2kmIzgyMjE7IGlzIGEgbm9uLXBvbGFyIFIgZ3JvdXAuIFlvdSBjYW4gdGVsbCBiZWNhdXNlIGl0IGhhcyBhIG1ldGh5bCBmdW5jdGlvbmFsIGdyb3VwLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBOb24tcG9sYXIgUiBncm91cHMgaGF2ZSBhIG1ldGh5bCBmdW5jdGlvbmFsIGdyb3VwIGF0dGFjaGVkIHRvIHRoZW0uIExvb2sgZm9yIHRoZSBSIGdyb3VwIHRoYXQgaGFzIGEgbWV0aHlsIGZ1bmN0aW9uYWwgZ3JvdXAgKOKAlENIMw==KS4=[Qq]
[/qwiz]
4.2. Proteins
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Proteins Quiz, APBVP”]
[h]Proteins
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14b0ca141e1d0e” question_number=”1″] In the diagram below, which number shows a ribosome synthesizing a protein?
[textentry single_char=”true”]
[c]ID c=[Qq]
[f]IEV4Y2VsbGVudC4gJiM4MjIwOzcmIzgyMjE7IHNob3dzIGEgcmlib3NvbWUgdHJhbnNsYXRpbmcgbVJOQSBpbnRvIGEgcG9seXBlcHRpZGUu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgcmlib3NvbWUgaXMgaW4gdGhlIGN5dG9wbGFzbSAodGhlIHJlZ2lvbiBkZXNpZ25hdGVkIGJ5IHRoZSBudW1iZXIgJiM4MjIwOzYuJiM4MjIxOw==[Qq]
[c]IEVudGVyIGxldHRlcg==[Qq]
[f]IE5vLg==[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14b0868ec5490e” question_number=”2″] In the diagram below, which number shows a newly synthesized polypeptide?
[textentry single_char=”true”]
[c]ID g=[Qq]
[f]IE5pY2Ugam9iLiAmIzgyMjA7OCYjODIyMTsgc2hvd3MgYSByaWJvc29tZSB0cmFuc2xhdGluZyBtUk5BIGludG8gYSBwb2x5cGVwdGlkZSwgYW5kIHRoYXQgcG9seXBlcHRpZGUgY2hhaW4gaXMgaW5kaWNhdGVkIGJ5IHRoZSBudW1iZXIgJiM4MjIwOzguJiM4MjIxOw==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgcmlib3NvbWUgYXQgbnVtYmVyICYjODIyMDs3LiYjODIyMTsgV2hhdCBpcyBpdCBzeW50aGVzaXppbmc/[Qq]
[c]IEVudGVyIGxldHRlcg==[Qq]
[f]IFNvcnJ5LCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14b043096c750e” question_number=”3″] In the diagram below, which number indicates the polypeptide backbone?
[textentry single_char=”true”]
[c]ID E=[Qq]
[f]IEdvb2Qgd29yay4gJiM4MjIwOzEmIzgyMjE7IGluZGljYXRlcyB0aGUgcG9seXBlcHRpZGUgYmFja2JvbmUgKGV2ZXJ5dGhpbmcgYnV0IHRoZSBzaWRlIGNoYWlucy4=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgcG9seXBlcHRpZGUgYmFja2JvbmUgaXMgZXZlcnl0aGluZyBidXQgdGhlIHNpZGUgY2hhaW5zIChhbHNvIGtub3duIGFzIFIgZ3JvdXBzKS4=[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14afff8413a10e” question_number=”4″] In the diagram below, which number shows an amino group?
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IENvcnJlY3QgLiAmIzgyMjA7MiYjODIyMTsgaW5kaWNhdGVzIGFuIGFtaW5vIGdyb3VwLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgYW1pbm8gZ3JvdXAgY29udGFpbnMgYSBuaXRyb2dlbiBhdG9tLg==[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14ad818f59990e” question_number=”13″] In the diagram below, the polypeptide backbone is indicated by number
[textentry single_char=”true”]
[c]ID E=[Qq]
[f]IFllcy4gVGhlIHBvbHlwZXB0aWRlIGJhY2tib25lIGlzIGluZGljYXRlZCBieSB0aGUgbnVtYmVyICYjODIyMDsxLiYjODIyMTs=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBMb29rIGZvciBhIG51bWJlciB0aGF0JiM4MjE3O3Mgbm90IHBvaW50aW5nIHRvIGFueSBvZiB0aGUgaW50ZXJhY3Rpbmcgc2lkZSBjaGFpbnMu[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14ad3bb5f4e10e” question_number=”14″] In the diagram below, a hydrogen bond between two side chains is indicated by number
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IE5pY2UuIE51bWJlciAmIzgyMjA7MiYjODIyMTsgc2hvd3MgdHdvIHNpZGUgY2hhaW5zIHRoYXQgYXJlIGh5ZHJvZ2VuIGJvbmRpbmcgd2l0aCBvbmUgYW5vdGhlciwgY2F1c2luZyBhIGJlbmQgaW4gdGhlIHBvbHlwZXB0aWRlIGJhY2tib25lLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IFNvcnJ5LCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBMb29rIGZvciBhIG51bWJlciB0aGF0JiM4MjE3O3Mgbm90IHBvaW50aW5nIHRvIGFueSBvZiB0aGUgaW50ZXJhY3Rpbmcgc2lkZSBjaGFpbnMu[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14acf8309c0d0e” question_number=”15″] In the diagram below, a disulfide bridge is indicated by number
[textentry single_char=”true”]
[c]ID M=[Qq]
[f]IFRlcnJpZmljLiBOdW1iZXIgJiM4MjIwOzMmIzgyMjE7IHNob3dzIGEgZGlzdWxmaWRlIGJyaWRnZS4=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBUaGluayBvZiB0aGUgcGhyYXNlICYjODIyMDtkaXN1bGZpZGUgYnJpZGdlLiYjODIyMTsgV2hhdCBlbGVtZW50IGRvIHlvdSB0aGluayBpcyBpbnZvbHZlZCBpbiB0aGlzIHR5cGUgb2YgYm9uZD8gTm93LCBsb29rIGZvciBhdG9tcyBvZiB0aGF0IGVsZW1lbnQu[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14acb25737550e” question_number=”16″] In the diagram below, hydrophobic clustering is indicated by number
[textentry single_char=”true”]
[c]ID Q=[Qq]
[f]IFdheSB0byBnbyEgTnVtYmVyICYjODIyMDs0JiM4MjIxOyBzaG93cyBoeWRyb3Bob2JpYyBjbHVzdGVyaW5nLg==[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IFNvcnJ5LCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBIeWRyb3Bob2JpYyBjbHVzdGVyaW5nIGludm9sdmVzIG5vbi1wb2xhciwgaHlkcm9waG9iaWMgc2lkZS1jaGFpbnMuIExvb2sgZm9yIHR3byBzaWRlIGNoYWlucyB0aGF0IGhhdmUgbm9uLXBvbGFyIGZ1bmN0aW9uYWwgZ3JvdXBzLCBhbmQgYXJlIGNsdXN0ZXJpbmcgdG9nZXRoZXIu[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14ac60d997290e” question_number=”17″] In the diagram below, a tertiary interaction involving ionic bonding is shown at
[textentry single_char=”true”]
[c]ID U=[Qq]
[f]IEdyZWF0ISBOdW1iZXIgJiM4MjIwOzUmIzgyMjE7IHNob3dzIGFuIGlvbmljIGJvbmQgYmV0d2VlbiBzaWRlIGNoYWlucy4=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IFNvcnJ5LCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50LiBJb25pYyBib25kcyBpbnZvbHZlIHRyYWRpbmcgZWxlY3Ryb25zLCB3aGljaCByZXN1bHRzIGluIGZ1bGx5IGNoYXJnZWQgYXRvbXMgb3IgZnVuY3Rpb25hbCBncm91cHMuIExvb2sgYXQgdGhlIGRpYWdyYW0gYWJvdmUsIGFuZCBmaW5kIHBvc2l0aXZlIGFuZCBuZWdhdGl2ZSBjaGFyZ2VzLCBhbmQgdGhlcmUgeW91JiM4MjE3O2xsIGZpbmQgYW4gaW9uaWMgYm9uZC4=[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14ac1b0032710e” question_number=”18″] In the diagram below, primary structure is indicated by number
[textentry single_char=”true”]
[c]ID E=[Qq]
[f]IEV4Y2VsbGVudC4gVGhlIHByaW1hcnkgc3RydWN0dXJlIGlzIGluZGljYXRlZCBieSB0aGUgbnVtYmVyICYjODIyMDsxLiYjODIyMTs=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBUaGUgcHJpbWFyeSBzdHJ1Y3R1cmUgaXMgdGhlIHNlcXVlbmNlIG9mIGFtaW5vIGFjaWRzLiBXaGljaCBudW1iZXIgc2hvd3MgdGhhdCBzZXF1ZW5jZSAod2l0aG91dCBhbnkgb2YgdGhlIGZvbGRpbmcgYW5kIGJlbmRpbmcgdGhhdCBlbWVyZ2VzIGF0IGhpZ2hlciBsZXZlbHMgb2Ygc3RydWN0dXJlPw==[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14abd77ad99d0e” question_number=”19″] In the diagram below, an alpha helix is indicated by number
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IFRlcnJpZmljLiBBbiBhbHBoYSBoZWxpeCAoYSBzZWNvbmRhcnkgbGV2ZWwgb2YgcHJvdGVpbiBzdHJ1Y3R1cmUpIGlzIHNob3duIGF0IG51bWJlciAmIzgyMjA7Mi4mIzgyMjE7[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBUaGUgc2Vjb25kYXJ5IHN0cnVjdHVyZSBpbnZvbHZlcyBoeWRyb2dlbiBib25kaW5nIGJldHdlZW4gdGhlIGFtaW5vIGFuZCBjYXJib255bCBmdW5jdGlvbmFsIGdyb3VwcyB3aXRoaW4gYSBwcm90ZWluJiM4MjE3O3MgcG9seXBlcHRpZGUgYmFja2JvbmUuIFR3byBwcmltYXJ5IHNlY29uZGFyeSBsZXZlbCBzdHJ1Y3R1cmVzIGFyZSBhbHBoYSBoZWxpY2VzIGFuZCBiZXRhLXBsZWF0ZWQgc2hlZXRzLiBUaGUgaGVsaXggaXMgbGlrZSBhIGNvcmtzY3JldyAod2hpY2ggc2hvdWxkIGhlbHAgeW91IGZpbmQgaXQpLg==[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14ab8aa551390e” question_number=”20″] In the diagram below, a protein’s tertiary structure is indicated by number
[textentry single_char=”true”]
[c]ID Q=[Qq]
[f]IE5pY2UuIFRlcnRpYXJ5IHN0cnVjdHVyZSBpcyBpbmRpY2F0ZWQgYnkgdGhlIG51bWJlciAmIzgyMjA7NC4mIzgyMjE7[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBUZXJ0aWFyeSBzdHJ1Y3R1cmUgaXMgd2hhdCBhcmlzZXMgYnkgYm9uZGluZyBiZXR3ZWVuIHNpZGUgY2hhaW5zIHdpdGhpbiBhIHNpbmdsZSBwb2x5cGVwdGlkZSBjaGFpbiwgd2hpY2ggcmVzdWx0cyBpbiBhIHZlcnkgY29tcGxleCB0aHJlZS1kaW1lbnNpb25hbCBzaGFwZS4=[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem_8_proteins_cumulative|14ab4023d4b90e” question_number=”21″] In the diagram below, a protein’s quaternary structure is indicated by number
[textentry single_char=”true”]
[c]ID U=[Qq]
[f]IFBlcmZlY3QuIFF1YXRlcm5hcnkgc3RydWN0dXJlIGlzIGluZGljYXRlZCBieSB0aGUgbnVtYmVyICYjODIyMDs1LiYjODIyMTs=[Qq]
[c]IEVudGVyIHdvcmQ=[Qq]
[f]IE5vLCB0aGF0JiM4MjE3O3Mgbm90IGNvcnJlY3Qu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBRdWF0ZXJuYXJ5IHN0cnVjdHVyZSBpcyB3aGF0IGFyaXNlcyBmcm9tIGludGVyYWN0aW9ucyBiZXR3ZWVuIG11bHRpcGxlIHBvbHlwZXB0aWRlIGNoYWlucy4gQm9uZHMgYmV0d2VlbiB0aGVzZSBjaGFpbnMgbGVhZCB0aGVtIHRvIGZvcm0gYSBtdWx0aS1wb2x5cGVwdGlkZSBjaGFpbiBwcm90ZWluLiBXaGljaCBudW1iZXIgc2hvd3MgbXVsdGlwbGUgcG9seXBlcHRpZGUgY2hhaW5zPw==[Qq]
[/qwiz]
What’s next?
Please proceed to this next tutorial in our AP Bio Video Pathway: Nucleic Acids
