1. Watch this Video
2. Study this Summary
The Key Elements of Life are Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, and Sulfur (CHNOPS).
- Carbon: Because carbon has four valence electrons, it can form a wide variety of covalent bonds, including single, double, and triple bonds with itself and other elements. This enables carbon to form rings, chains, and branched molecules of any length and shape. This makes possible the formation of the complex molecules that are the basis of life.
- Hydrogen: Plays a role in energy storage and exchange, creating acidic micro-enviroments, and creating proton gradients during the process of chemiosmosis (a form of ATP production covered in AP Bio Unit 3)
- Nitrogen: Nitrogenous bases are key to the structure of DNA and RNA. Nitrogen-containing amino groups are key to the structure of proteins.
- Oxygen: Plays a key role in cellular respiration
- Phosphorus: Found in phosphate groups (e.g., ATP). Phosphate groups are key to the structure of phospholipids (which make up cell membranes) and in nucleic acids (DNA and RNA).
- Sulfur: plays a key role in protein structure
Monomers, Polymers, and Functional Groups
Monomers and Polymers
- Three groups of biological macromolecules (carbohydrates, proteins, and nucleic acids) are polymers, built from smaller units called monomers.
- Analogy: Monomers are like Lego blocks, while polymers are the structures built from them.
Dehydration Synthesis
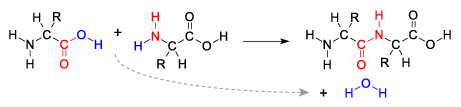
- Process of joining monomers to form polymers by removing a water molecule (H2O).
- Enzymes facilitate the reaction by removing a hydroxyl group from one monomer and a hydrogen from the other.
Hydrolysis
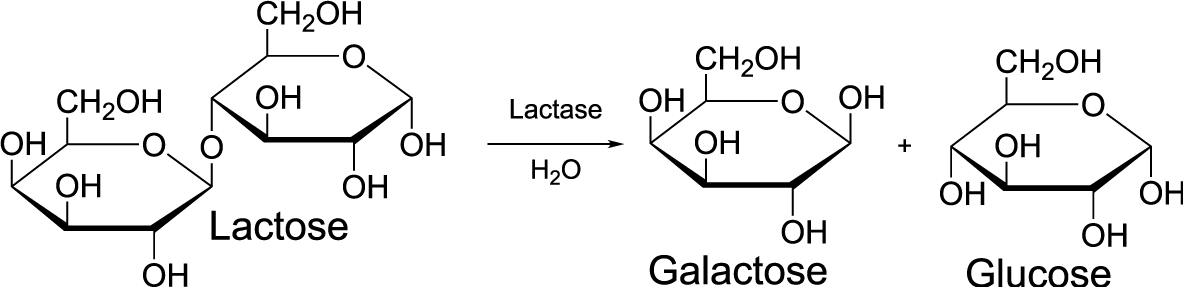
- Opposite of dehydration synthesis; breaks polymers into monomers by inserting a water molecule between the monomers.
- Example: Enzymes break apart lactose (a disaccharide) into two carbohydrate monomers: glucose and galactose by inserting a water molecule between the two monomers.
Functional Groups
Not directly tested on the AP Bio exam but critical for understanding molecular structures (and they might be on your teacher’s test).
- Phosphate group: Key in energy exchange (e.g., ATP) and DNA structure.
- Methyl group: Enzymes use methyl groups to silence DNA (methylation).
- Hydroxyl and carbonyl groups make molecules hydrophilic (able to dissolve in water).
- Carboxyl group (carboxylic acid): Present in amino acids and other molecules, making them acidic
- Amino group: Found in amino acids. Makes molecules basic.
- Sulfhydryl group: Stabilizes protein structures.
- Acetyl group: Found in a molecule that’s important in cellular respiration (Acetyl-CoA). Adding acetyl groups to DNA-associated proteins (acetylation) is used to activates DNA for gene expression (the opposite of methylation).
3. Master these Flashcards
[qdeck bold_text=”false” qrecord_id=”sciencemusicvideosMeister1961-Biochemistry Basic Concepts, APBVP”]
[h]Biochemistry Basic Concepts
[i]
[!]1.2.Elements of Life[/!]
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” dataset_id=”AP_Bio_Flashcards_2022|1fe2c67d4bd10″ question_number=”6″ topic=”1.2.Elements_of_Life”] Describe the biological importance of carbon, and explain why carbon plays the role that it does in living things.
[a] Carbon is the central atom in biological molecules. Because carbon has four valence electrons, it can form a wide variety of covalent bonds, including single, double, and triple bonds with itself and other elements. This enables carbon to form rings, chains, and branched molecules of any length and shape. This makes possible the formation of complex molecules that, at a molecular level, underlie life’s properties: replication, energy transfer, encapsulation, etc.
[q json=”true” yy=”4″ dataset_id=”AP_Bio_Flashcards_2022|1fd8e14ac2d10″ question_number=”7″ unit=”1.Chemistry_of_Life” topic=”1.2.Elements_of_Life”] Explain the biological importance of hydrogen.
[a] Hydrogen is a key atom in almost every biological molecule. One of its key roles is transferring chemical energy, as when the electron carrier NAD+ gains energy during its reduction to NADH. Hydrogen ions (or protons) are used to create acidic environments within cellular compartments like lysosomes, or in body cavities like the stomach. Protons also play a key role in one of life’s most basic energy processes, chemiosmosis, which is used to create ATP during both photosynthesis and cellular respiration.
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” dataset_id=”AP_Bio_Flashcards_2022|1fceb196bd510″ question_number=”8″ topic=”1.2.Elements_of_Life”] Describe the biological importance of nitrogen.
[a] Nitrogen is a key part of proteins, making up a central part of the amine group found in every amino acid. Nitrogen is also in the nitrogenous bases that make up nucleotides. Note that one of these nitrogen-bearing nucleotides is ATP, life’s energy transfer molecule.
Nitrogen can be a key limiting factor in ecosystems.
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” dataset_id=”AP_Bio_Flashcards_2022|1fc3329c07910″ question_number=”9″ topic=”1.2.Elements_of_Life”] Describe the biological importance of oxygen.
[a] Oxygen is a key part of almost all biological molecules. Oxygen is required for aerobic cellular respiration, during which oxygen acts as the final electron acceptor.
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” dataset_id=”AP_Bio_Flashcards_2022|1faf6836f5910″ question_number=”11″ topic=”1.2.Elements_of_Life”] Describe the biological importance of phosphorus.
[a] Phosphorus, as part of a phosphate group (-PO4), plays roles in energy transfer and information transfer. Living things energize molecules (such as ADP) by adding phosphate groups to them (creating, in this example, ATP) . Transferring phosphate groups releases energy that can power cellular work (which occurs when ATP is broken down to ADP and Pi (inorganic phosphate). Phosphate groups are part of the sugar-phosphate backbone of life’s key informational molecules, DNA and RNA. Phosphate groups also play a key role in the structure of phospholipids, the key structural molecules in cell membranes.
Phosphorus (like nitrogen) is often a key limiting factor in ecosystems
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” dataset_id=”AP_Bio_Flashcards_2022|1fa55dc3ae510″ question_number=”12″ topic=”1.2.Elements_of_Life”] Describe the biological importance of sulfur.
[a] Sulfur is a key part of the structure of proteins.
[q json=”true” yy=”4″ unit=”1.Chemistry_of_Life” dataset_id=”AP_Bio_Flashcards_2022|1f9b535067110″ question_number=”13″ topic=”1.3.Monomers_and_Polymers”] What are monomers? How do they connect to form polymers?
[a] Three of the four macromolecule families that make up living things — carbohydrates, proteins, and nucleic acids — are built from smaller building blocks called monomers. These monomers are, respectively, monosaccharides, amino acids, and nucleotides. Living things build macromolecules with specific three-dimensional shapes and functions by combining monomers into polymers through a process called dehydration synthesis.
[q json=”true” dataset_id=”AP_Bio_Flashcards_2022|2299716d4b0319″ question_number=”14″ unit=”1.Chemistry_of_Life” topic=”1.3.Monomers_and_Polymers”] Explain dehydration synthesis.
[a] Living things combine monomers into polymers through enzyme-catalyzed dehydration synthesis reactions. In these reactions, a hydroxyl group (an —OH) is pulled off of one monomer (or a group of already connected monomers), and a hydrogen atom is pulled off the other. The —H and —OH combine to form water (hence, dehydration synthesis).
[q json=”true” dataset_id=”AP_Bio_Flashcards_2022|22988d40bdbb19″ question_number=”15″ unit=”1.Chemistry_of_Life” topic=”1.3.Monomers_and_Polymers”] Describe hydrolysis.
[a] Living things digest or recycle polymers through hydrolysis (“breaking with water”). Enzymes insert a water molecule between the monomers making up the polymer. This breaks the bond that held the two monomers together.
[q json=”true” dataset_id=”AP_Bio_Flashcards_2022|added1″ question_number=”16″ unit=”1.Chemistry_of_Life” topic=”1.3.Functional_Groups”] Describe the role of the methyl group in molecular biology.
[a] The methyl group (-CH3) is used by enzymes to silence DNA through a process called methylation. This makes DNA less accessible for transcription, regulating gene expression.
[q json=”true” dataset_id=”AP_Bio_Flashcards_2022|added2″ question_number=”17″ unit=”1.Chemistry_of_Life” topic=”1.3.Functional_Groups”] What is the function of the acetyl group in cellular respiration and gene expression?
[a] The acetyl group is part of acetyl-CoA, a key molecule in cellular respiration that feeds carbon atoms into the Krebs cycle (which you’ll learn about in AP Bio Unit 3). Acetylation of DNA-associated proteins activates genes for expression.
[q json=”true” dataset_id=”AP_Bio_Flashcards_2022|added3″ question_number=”18″ unit=”1.Chemistry_of_Life” topic=”1.3.Functional_Groups”] How do hydroxyl and carbonyl groups affect molecules?
[a] Hydroxyl (-OH) and carbonyl (C=O) groups make molecules polar and hydrophilic, enabling them to dissolve in water and interact with other polar substances.
[x]
[restart]
[/qdeck]
4. Tackle these Quizzes
4.1. Carbon and the Elements of Life
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Carbon and the Elements of Life, APBVP”]
[h]Carbon and the Elements of Life
[i]
CHNOPS
[q]Because carbon has four outer electrons, it can be organized into a variety of forms. Label these forms in the table below.
| ______________ | _____________ | ____________ |
[l]branches
[f*] Good!
[fx] No. Please try again.
[l]chains
[f*] Great!
[fx] No. Please try again.
[l]rings
[f*] Excellent!
[fx] No. Please try again.
[q]The central atom in phosphate groups, which are found in ATP, DNA, and RNA.
[hangman]
[c]cGhvc3Bob3J1cw==[Qq]
[q]Glucose is energy-rich because of this element.
[hangman]
[c]aHlkcm9nZW4=[Qq]
[q]This element is part of a sulfhydryl functional group. It’s found in two of the twenty amino acids that make up proteins, and required for protein structure.
[hangman]
[c]c3VsZnVy[Qq]
[q]The central element in living things.
[hangman]
[c]Y2FyYm9u[Qq]
[q]A six-letter acronym that you can use to remember the six key elements in living things.
[hangman]
[c]Q0hOT1BT[Qq]
[/qwiz]
4.2. Functional groups
Note that some AP Biology teachers emphasize functional groups, while others don’t. My perspective is that they come up again and again in AP Bio, and it’s useful to be able to identify functional groups and to know which ones are polar, non-polar, acidic, basic, and so on.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Functional Groups, APBVP”]
[h]Functional groups
[i]
[q]In the diagram below, a hydroxyl group is shown at
[textentry single_char=”true”]
[c]ID U=[Qq]
[f]IE5pY2Ugam9iOiBhIGh5ZHJveHlsIGdyb3VwIGlzIGF0ICYjODIyMDs1LiYjODIyMTs=[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgaHlkcm94eWwgZ3JvdXAgaXMgbGlrZSBhIHdhdGVyIG1vbGVjdWxlLCBidXQgaXQmIzgyMTc7cyBtaXNzaW5nIGEgaHlkcm9nZW4gYXRvbS4=
Cg==[Qq]
[q]In the diagram below, a methyl group is shown at
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IE5pY2Ugam9iOiBhIG1ldGh5bMKgZ3JvdXAgaXMgYXQgJiM4MjIwOzIuJiM4MjIxOw==[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgbWV0aHlswqBncm91cCBpcyBsaWtlIGEgbWV0aGFuZcKgbW9sZWN1bGUsIChDSA==NA==KSwgYnV0IGl0JiM4MjE3O3MgbWlzc2luZyBhIGh5ZHJvZ2VuIGF0b20u
Cg==[Qq]
[q]In the diagram below, a phosphate group is shown at
[textentry single_char=”true”]
[c]wq Ax[Qq]
[f]IE5pY2Ugam9iOiBhIHBob3NwaGF0ZcKgZ3JvdXAgaXMgYXQgJiM4MjIwOzEuJiM4MjIxOw==[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgcGhvc3BoYXRlwqBncm91cCBpcyB0aGUgb25seSBvbmUgd2l0aCBwaG9zcGhvcnVzLg==
Cg==[Qq]
[q]In the diagram below, a sulfhydryl group is shown at
[textentry single_char=”true”]
[c]wq A2[Qq]
[f]IE5pY2Ugam9iOiBhIHN1bGZoeWRyeWzCoGdyb3VwIGlzIGF0ICYjODIyMDs2LiYjODIyMTs=[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBMb29rIGF0IHRoZSBuYW1lIG9mIHRoaXMgZnVuY3Rpb25hbCBncm91cCAoJiM4MjIwO3N1bGZoeWRyeWwmIzgyMjE7KS4gVGhlIGZpcnN0IHN5bGxhYmxlIHRlbGxzIHlvdSB3aGljaCBlbGVtZW50IHRvIGxvb2sgZm9yLg==
Cg==[Qq]
[q]In the diagram below, a carboxyl group is shown at
[textentry single_char=”true”]
[c]wq Az[Qq]
[f]IE5pY2Ugam9iOiBhIGNhcmJveHlswqBncm91cCBpcyBhdCAmIzgyMjA7My4mIzgyMjE7[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlIGFyZSB0d28gaGludHMuIDEpIFRoZSBuYW1lICYjODIyMDtjYXJib3h5bCYjODIyMTsgaW5kaWNhdGVzIG9uZSBvZiB0aGUga2V5IGVsZW1lbnRzIGluIHRoaXMgZ3JvdXAuIDIpIFRoZSBjYXJib3h5bCBncm91cCBpcyBvbmUgb2YgdGhlIGZ1bmN0aW9uYWwgZ3JvdXBzIHRoYXQgY2FuIGJlIGluIGFuIGlvbml6ZWQgZm9ybSAobWVhbmluZyB0aGF0IGl0IHdpbGwgaGF2ZSBhIGNoYXJnZSAoaW5kaWNhdGVkIGJ5IGEgJiM4MjIwO3BsdXMmIzgyMjE7IG9yIGEgJiM4MjIwO21pbnVzJiM4MjIxOyBzaWduKS4gU28sIGxvb2sgZm9yIHRoZSBlbGVtZW50IEkmIzgyMTc7dmUgaGludGVkIGF0LCBhbmQgYSBjaGFyZ2Uu
Cg==[Qq]
[q]In the diagram below, a carbonyl group is shown at
[textentry single_char=”true”]
[c]wq A3[Qq]
[f]IE5pY2Ugam9iOiBhIGNhcmJvbnlswqBncm91cCBpcyBhdCAmIzgyMjA7Ny4mIzgyMjE7[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGUgY2FyYm9ueWzCoGdyb3VwJiM4MjE3O3MgbmFtZSBpbmRpY2F0ZXMgb25lIG9mIGl0cyBtb3N0IGltcG9ydGFudCBlbGVtZW50cy4=
Cg==[Qq]
[q]In the diagram below, an amino group is shown at
[textentry single_char=”true”]
[c]wq A0[Qq]
[f]IE5pY2Ugam9iOiBhbiBhbWlubyBncm91cCBpcyBhdCAmIzgyMjA7NC4mIzgyMjE7[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGXCoGZvdXJ0aCBsZXR0ZXIgaW4gdGhlIHdvcmQgJiM4MjIwO2FtaW5vJiM4MjIxOyBpbmRpY2F0ZXMgYSBrZXkgZWxlbWVudCBpbiB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAu
Cg==[Qq]
[q]In the diagram below, acetyl is shown at
[textentry single_char=”true”]
[c]wq A4[Qq]
[f]IE5pY2Ugam9iOiBhbiBhY2V0eWwgZ3JvdXAgaXMgYXQgJiM4MjIwOzguJiM4MjIxOw==[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBBY2V0eWwgZ3JvdXBzIGhhdmUgYSBtZXRoeWwgZ3JvdXAgYW5kIGEgY2FyYm9ueWwgZ3JvdXAu
Cg==[Qq]
[q]In the diagram below, the functional group that’s used to deactivate stretches of DNA (turning genes off) is at
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IE5pY2Ugam9iOiBhIG1ldGh5bMKgZ3JvdXAgaXMgYXQgJiM4MjIwOzIuJiM4MjIxOyBUaGVzZSBncm91cHMgYXJlIG9mdGVuIHVzZWQgaW4gZ2VuZSBpbmFjdGl2YXRpb24u[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgVGhpcyBwcm9jZXNzIG9mIHR1cm5pbmcgZ2VuZXMgb2ZmIGJ5IGFkZGluZyBhIGZ1bmN0aW9uYWwgZ3JvdXAgaXMgY2FsbGVkICYjODIyMDttZXRoeWxhdGlvbi4mIzgyMjE7
Cg==[Qq]
[q]In the diagram below, the functional group that’s often used in energy transfer is at
[textentry single_char=”true”]
[c]wq Ax[Qq]
[f]IE5pY2Ugam9iOiBhIHBob3NwaGF0ZcKgZ3JvdXAgaXMgc2hvd24gYXQgJiM4MjIwOzEuJiM4MjIxOyBUaGVzZSBhcmUgb2Z0ZW4gdXNlZCBpbiBlbmVyZ3kgdHJhbnNmZXIgKGFzIGluIHRoZSBtb2xlY3VsZSBhZGVub3NpbmUgdHJpcGhvc3BoYXRlLCBBVFApLg==[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LiBUaGVzZSB0eXBlcyBvZiBlbmVyZ3kgdHJhbnNmZXJzIGFyZSByZWZlcnJlZCB0byBhcyA=cGhvc3Bob3J5bGF0aW9ucw==Lg==
Cg==[Qq]
[q]In the diagram below, the functional group that plays a key role in protein structure through the formation of disulfide bridges is
[textentry single_char=”true”]
[c]wq A2[Qq]
[f]IE5pY2Ugam9iOiBhIHN1bGZoeWRyeWzCoGdyb3VwIGlzIHNob3duIGF0ICYjODIyMDs2LiYjODIyMTsgVHdvIHN1bGZoeWRyeWwgZ3JvdXBzIGNhbiBmb3JtIGEgc3RydWN0dXJlIGNhbGxlZCBhICYjODIyMDtkaXN1bGZpZGUmIzgyMjE7IGJyaWRnZSwgd2hpY2ggY2FuIGJlbmQgYSBwcm90ZWluIGludG8gYSBwYXJ0aWN1bGFyIHNoYXBlICh3aGljaCBoZWxwcyB0byBkZXRlcm1pbmUgdGhlIHByb3RlaW4mIzgyMTc7cyBzaGFwZSwgd2hpY2ggaW4gdHVybiBoZWxwcyBkZXRlcm1pbmUgaXRzIGZ1bmN0aW9uKS4=[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgVGhlIHN0cnVjdHVyZSBpcyBjYWxsZWQgYSAmIzgyMjA7ZGlzdWxmaWRlIGJyaWRnZS4mIzgyMjE7IFdoYXQgZWxlbWVudCBuYW1lIGRvIHlvdSBzZWUgaW4gJiM4MjIwO2Rpc3VsZmlkZS4mIzgyMjE7IFdoaWNoIGZ1bmN0aW9uYWwgZ3JvdXAgaGFzIHRoYXQgZWxlbWVudD8=
Cg==[Qq]
[q]Note that in this question, the diagram is just for reference. The two functional groups that can make the molecule they’re attached to acidic are
[c]bWV0aHlsIGFuZCBjYXJib255bA==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgTG9vayBmb3IgdGhlIHR3byBmdW5jdGlvbmFsIGdyb3VwcyB0aGF0LCBpbiB0aGVpciBpb25pemVkIGZvcm0sIGhhdmUgbmVnYXRpdmUgY2hhcmdlcy4gVGhleSYjODIxNztyZSBuZWdhdGl2ZSBiZWNhdXNlIHRoZXkgbG9zdCBhIGh5ZHJvZ2VuIGlvbiwgd2hpY2ggaGFzIGRpc3NvbHZlZCBpbnRvIHRoZSBzdXJyb3VuZGluZyBzb2x1dGlvbiwgbWFraW5nIHRoYXQgc29sdXRpb24gbW9yZSBhY2lkaWMu[Qq]
[c]wqBwaG9zcGhhdGUgYW5kIGFtaW5v[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgTG9vayBmb3IgdGhlIHR3byBmdW5jdGlvbmFsIGdyb3VwcyB0aGF0LCBpbiB0aGVpciBpb25pemVkIGZvcm0sIGhhdmUgbmVnYXRpdmUgY2hhcmdlcy4gVGhleSYjODIxNztyZSBuZWdhdGl2ZSBiZWNhdXNlIHRoZXkgbG9zdCBhIGh5ZHJvZ2VuIGlvbiwgd2hpY2ggaGFzIGRpc3NvbHZlZCBpbnRvIHRoZSBzdXJyb3VuZGluZyBzb2x1dGlvbiwgbWFraW5nIHRoYXQgc29sdXRpb24gbW9yZSBhY2lkaWMu[Qq]
[c]c3VsZmh5ZHJ5bMKgYW5kIGNhcmJveHls[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgTG9vayBmb3IgdGhlIHR3byBmdW5jdGlvbmFsIGdyb3VwcyB0aGF0LCBpbiB0aGVpciBpb25pemVkIGZvcm0sIGhhdmUgbmVnYXRpdmUgY2hhcmdlcy4gVGhleSYjODIxNztyZSBuZWdhdGl2ZSBiZWNhdXNlIHRoZXkgbG9zdCBhIGh5ZHJvZ2VuIGlvbiwgd2hpY2ggaGFzIGRpc3NvbHZlZCBpbnRvIHRoZSBzdXJyb3VuZGluZyBzb2x1dGlvbiwgbWFraW5nIHRoYXQgc29sdXRpb24gbW9yZSBhY2lkaWMu[Qq]
[c]Y2FyYm94eWwgYW5k IHBob3NwaGF0ZQ==[Qq]
[f]IE5pY2Ugam9iOiBUaGUgdHdvIGZ1bmN0aW9uYWwgZ3JvdXBzIHRoYXQgbWFrZSB0aGUgbW9sZWN1bGUgdGhleSYjODIxNztyZSBhdHRhY2hlZCB0byBhY2lkaWMgYXJlIHBob3NwaGF0ZSBhbmQgY2FyYm94eWwu
Cg==Jm5ic3A7
Cg==[Qq]
[q]In the diagram below, the functional group that can make the molecule it’s attached to a base is
[textentry single_char=”true”]
[c]wq A0[Qq]
[f]IE5pY2Ugam9iOsKgdGhlIGFtaW5vIGdyb3VwLCBhdCA0LCBjYW4gYWJzb3JiIGEgaHlkcm9nZW4gaW9uIGZyb20gdGhlIHNvbHV0aW9uIGl0JiM4MjE3O3MgaW4sIG1ha2luZyB0aGUgc29sdXRpb24gbW9yZSBiYXNpYy4=[Qq]
[c]Kg==[Qq]
[f]Tm8sIGJ1dCBoZXJlJiM4MjE3O3MgYSBoaW50LsKgQW1tb25pYSBpcyBhIGJhc2UgdGhhdCBoYXMgYSBzdHJ1Y3R1cmUgcmVsYXRlZCB0byB0aGlzIGZ1bmN0aW9uYWwgZ3JvdXAuIEFtbW9uaWEmIzgyMTc7cyBmaWZ0aCBsZXR0ZXIgaGFzIGFuIGVsZW1lbnQgdGhhdCB3aWxsIGxlYWQgeW91IHJpZ2h0IHRvIHRoaXMgZnVuY3Rpb25hbCBncm91cC4=[Qq]
[/qwiz]
4.3 Monomers and Polymers
[qwiz random = “false” qrecord_id=”sciencemusicvideosMeister1961-Monomers and Polymers, APBVP”]
[h]Monomers and Polymers
[i]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem1_Monomers and Polymers|1e951f6cb4795b” question_number=”7″] [hangman] get combined with one another to form polymers.
[c]IE1vbm9tZXJz[Qq]
[f]IENvcnJlY3Qh[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem1_Monomers and Polymers|1e94e9dfa2fd5b” question_number=”8″] The molecule shown below (glucose) is one of the [hangman] of carbohydrates.
[c]IG1vbm9tZXJz[Qq]
[f]IENvcnJlY3Qh[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem1_Monomers and Polymers|1e94b1fe859d5b” question_number=”9″] The repeating glucose subunits in starch make starch a [hangman].
[c]IHBvbHltZXI=[Qq]
[q json=”true” xx=”2″ dataset_id=”SMV_biochem1_Monomers and Polymers|1e946e792cc95b” question_number=”10″] Letters are to monomers as words are to [hangman].
[c]IHBvbHltZXJz[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem1_Monomers and Polymers|1e9421a3a4655b” question_number=”11″] The reaction below is an example of
[c]IGh5ZHJvbHlzaXM=[Qq]
[f]IE5vLiBIeWRyb2x5c2lzIHdvdWxkIGludm9sdmUgYnJlYWtpbmcgbW9ub21lcnMgYXBhcnQgZnJvbSBvbmUgYW5vdGhlci4gSGVyZSB0aGV5JiM4MjE3O3JlIGJlaW5nIGNvbWJpbmVkICh3aXRoIHRoZSByZW1vdmFsIG9mIGEgd2F0ZXIgbW9sZWN1bGUp[Qq]
[c]IGRlaHlkcmF0aW9u IHN5bnRoZXNpcw==[Qq]
[f]IFBlcmZlY3QuIFRoaXMgaXMgYSBkZWh5ZHJhdGlvbiBzeW50aGVzaXMgcmVhY3Rpb24u[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem1_Monomers and Polymers|1e93e07257755b” question_number=”12″] The reaction below is an example of
[c]IGh5ZHJv bHlzaXM=[Qq]
[f]IE5pY2Ugam9iLiBIeWRyb2x5c2lzIGludm9sdmVzIGJyZWFraW5nIG1vbm9tZXJzIGFwYXJ0IGZyb20gb25lIGFub3RoZXIgd2hpbGUgaW5zZXJ0aW5nIGEgd2F0ZXIgbW9sZWN1bGUgaW50byB0aGUgYm9uZCB0aGF0IHdhcyBmb3JtZXJseSBob2xkaW5nIHRoZW0gdG9nZXRoZXIuIFRoYXQmIzgyMTc7cyBleGFjdGx5IHdoYXQgeW91IHNlZSBoZXJlLg==[Qq]
[c]IGRlaHlkcmF0aW9uIHN5bnRoZXNpcw==[Qq]
[f]IE5vLiBEZWh5ZHJhdGlvbiBzeW50aGVzaXMgaW52b2x2ZXMgY29tYmluaW5nIG1vbm9tZXJzIGJ5IHJlbW92aW5nIGEgd2F0ZXIgbW9sZWN1bGUu[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem1_Monomers and Polymers|1e939cecfea15b” question_number=”13″] The reaction below is an example of
[c]IGh5ZHJvbHlzaXM=[Qq]
[f]IE5vLiBIeWRyb2x5c2lzIHdvdWxkIGludm9sdmUgYnJlYWtpbmcgbW9ub21lcnMgYXBhcnQgZnJvbSBvbmUgYW5vdGhlci4gSGVyZSB0d28gYW1pbm8gYWNpZHMgYXJlIGJlaW5nIGNvbWJpbmVkICh3aXRoIHRoZSByZW1vdmFsIG9mIGEgd2F0ZXIgbW9sZWN1bGUp[Qq]
[c]IGRlaHlkcmF0aW9u IHN5bnRoZXNpcw==[Qq]
[f]IFBlcmZlY3QuIFRoaXMgaXMgYSBkZWh5ZHJhdGlvbiBzeW50aGVzaXMgcmVhY3Rpb24uIFR3byBhbWlubyBhY2lkcyBhcmUgY29tYmluZWQsIHdpdGggdGhlIHJlbW92YWwgb2YgYSB3YXRlciBtb2xlY3VsZS4=[Qq]
[q json=”true” multiple_choice=”true” xx=”2″ dataset_id=”SMV_biochem1_Monomers and Polymers|1e93183658dd5b” question_number=”14″] The reaction below is an example of
[c]IGh5ZHJv bHlzaXM=[Qq]
[f]IE5pY2Ugam9iLiBIeWRyb2x5c2lzIGludm9sdmVzIGJyZWFraW5nIG1vbm9tZXJzIGFwYXJ0IGZyb20gb25lIGFub3RoZXIgd2hpbGUgaW5zZXJ0aW5nIGEgd2F0ZXIgbW9sZWN1bGUgaW50byB0aGUgYm9uZCB0aGF0IHdhcyBmb3JtZXJseSBob2xkaW5nIHRoZW0gdG9nZXRoZXIuIFRoYXQmIzgyMTc7cyBleGFjdGx5IHdoYXQgeW91IHNlZSBoZXJlLCBhcyBsYWN0b3NlIGlzIGJlaW5nIGJyb2tlbiBhcGFydCBpbnRvIGl0cyBtb25vbWVycywgZ2FsYWN0b3NlIGFuZCBnbHVjb3NlLg==[Qq]
[c]IGRlaHlkcmF0aW9uIHN5bnRoZXNpcw==[Qq]
[f]IE5vLiBEZWh5ZHJhdGlvbiBzeW50aGVzaXMgaW52b2x2ZXMgY29tYmluaW5nIG1vbm9tZXJzIGJ5IHJlbW92aW5nIGEgd2F0ZXIgbW9sZWN1bGUuIFdoYXQgeW91IHNlZSBhYm92ZSBpcyB0aGUgc3BsaXR0aW5nIGFwYXJ0IG9mIGEgbW9sZWN1bGUgdG8gcmVsZWFzZSB0d28gbW9ub21lcnMu[Qq]
[x][restart]
[/qwiz]
What’s next?
Please proceed to this next tutorial: Carbohydrates and Lipids
