1. Introduction
Living things, by weight, are mostly made of water. In other tutorials, we’ve looked at how water’s polarity and the hydrogen bonding that results affects water’s properties. In this tutorial, we’re going to look at another feature of water’s chemistry: acids, bases, and the pH scale.
 2. Solutions, Acids, and Bases
2. Solutions, Acids, and Bases
When you dissolve sugar, salt, or lemonade in water, you’ve made a solution. In a solution, one substance, the solute, is dissolved in another substance, the solvent. In the solution shown in the image to your left, the solute is sugar, and the solvent is water.
In this tutorial, we’re going to focus on two special types of solutions. Acidic solutions have a sour taste. If you have a cut on your skin, and you put an acidic solution on it, it will sting. Strong acids can cause burns, and even dissolve metals. Almost all fruit juices and sodas are acidic. Sulfuric acid and hydrochloric acid are two strong acids that you might have heard of.
Basic solutions taste bitter. Strong bases have a soapy feel when you touch them. Unlike acids, bases don’t dissolve metals, so they can be used to unclog pipes by dissolving the hair that might be clogging them, without dissolving the pipe. Many bleaches and cleaning agents are bases. The word “alkaline” means the same as “basic.”
To see if you’ve mastered the concepts above, take the quiz below.
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Solutions, Acids, Bases Quiz (M4)”]
[h]Solutions, Acids, and Bases
[i]The following questions include labeling, multiple-choice, and fill-in-the-blanks.
[q]A liquid mixture in which one thing is dissolved in another is a [hangman].
[c]c29sdX Rpb24=
Cg==[Qq]
[q]The thing that does the dissolving in a solution is the [hangman].
[c]c29sdm VudA==
Cg==[Qq]
[q]The thing that gets dissolved in a solution is the [hangman].
[c]c29s dXRl
Cg==[Qq]
[q]A solution with a bitter taste is a(n) [hangman].
[c]YmFz ZQ==
Cg==[Qq]
[q]A solution with a sour taste is a(n) [hangman].
[c]YWNp ZA==
Cg==[Qq]
[q][hangman] can dissolve metals
[c]YWNp ZHM=
Cg==[Qq]
[q][hangman] sting when you get them on a cut
[c]YWNp ZHM=[Qq]
[q]Soda drinks are _________
[c]YWNp ZGlj[Qq]
[c]YmFzaWM=[Qq]
[f]TmljZS7CoFNvZGEgZHJpbmtzIGFyZSBhY2lkaWMu[Qq]
[f]Tm8uIFNvZGEgZHJpbmtzIGhhdmUgYSBzb3VyIHRhc3RlLiBTb3VyIGxpcXVpZHMgYXJlIF9fX19fXy4=
Cg==[Qq]
[q][hangman] have a bitter taste.
[c]YmFz ZXM=
Cg==[Qq]
[q][hangman] have a slippery or soapy feel
[c]YmFz ZXM=[Qq]
[q labels = “right”]
[l]solvent
[fx] No. Please try again.
[f*] Great!
[l]solute
[fx] No. Please try again.
[f*] Great!
[q]A word that means the same as basic is [hangman].
[c]YWxrYW xpbmU=[Qq]
[x][restart]
[/qwiz]
3. The Chemistry of Acids and Bases: What Biology Students Need to Know
What causes a solution to be acidic or basic? A water molecule, or H20, can dissociate, or fall apart, to form two charged particles or ions. One of these ions is called a “hydrogen ion,” written as H+ (and pronounced “H-plus”). An H+ is simply a proton, floating around in a solution. The other ion is written as OH– (which is pronounced as “O” “H” minus). The OH– ion is also called “hydroxide.” Here’s the equation for the dissociation of water.
H2O → H+ + OH–
In a chemistry class, you’ll learn that the H+ immediately combines with a water molecule to form H3O+, also known as the hydronium ion. For biology students, it’s very useful to continue to think of water as dissociating into H+ and OH–
If dissolving something in water causes it to have more H+ than OH–, then the solution is acidic. For example, if the compound HCl (hydrochloric acid) is dissolved in water, the hydrogen ions dissociate from the chlorine as shown below:
HCl→H+ + Cl–
The added H+ ions make the resulting solution an acid.
Conversely, if dissolving something in water causes it to have more OH– than H+, then the resulting solution is a base. For example, when Sodium Hydroxide (lye) is dissolved in water, the sodium dissociates from the hydroxide ion:
NaOH → Na+ + OH–
If the amount H+ is equal to OH–, then the solution is neutral. Pure water has a pH of 7, but what comes out of the tap is usually a weak acid.
Acidic and basic solutions are measured on the pH (pronounced “P” “H”) scale. On the pH scale, the acids with the most hydrogen ions are at 0 (or rarely, below 0). The most concentrated bases are at pH 14 (or above). A neutral liquid is 7.
The scale works by powers of 10, so a solution with pH 5 is 10 times more acidic than a solution that is pH 6. A solution that is pH 12 is 10 times more basic than a solution that is pH 11.
Here’s a graphical version of the pH scale.
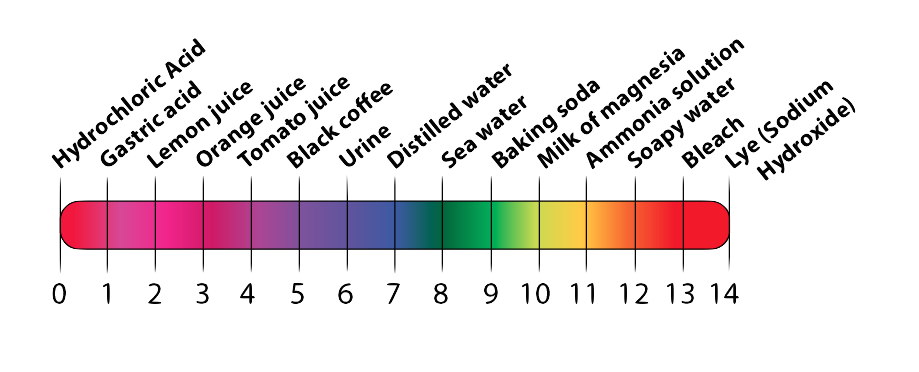
4. Acids, Bases, and pH: Checking Understanding
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Acids, Bases, pH Quiz (M4)”]
[h]Quiz: Acids, Bases and pH
[i]
[q]The change of a water molecule into an H+ and an OH– is called [hangman].
[c]ZGlzc29jaWF0aW9u
Cg==[Qq]
[q]Another word for the H+ ion is [hangman] ion.
[c]aHlkcm9nZW4=
Cg==[Qq]
[q]A solution with more H+ than OH– is a(n) [hangman].
[c]YWNpZA==
Cg==[Qq]
[q]A solution with more than OH– than H+ is a(n) [hangman].
[c]YmFzZQ==
Cg==[Qq]
[q]A solution with pH 9 is a(n) [hangman].
[c]YmFzZQ==
Cg==[Qq]
[q]A solution with pH 4 is a(n) [hangman].
[c]YWNpZA==
Cg==[Qq]
[q]A solution with pH 7 is
[c]YWNpZGlj[Qq]
[c]YmFzaWM=[Qq]
[c]bmV1dH JhbA==[Qq]
[f]Tm8uIFRha2UgYSBsb29rIGF0IHRoaXMgZGlhZ3JhbSwgYW5kIG5vdGljZSB3aGVyZSA3IGxpZXMu
Cg==[Qq]
[f]Tm8uIFRha2UgYSBsb29rIGF0IHRoaXMgZGlhZ3JhbSwgYW5kIG5vdGljZSB3aGVyZSA3IGxpZXMu
Cg==[Qq]
[f]Q29ycmVjdMKgLkHCoHNvbHV0aW9uwqBwSCA3wqBpc8KgbmV1dHJhbC4=
Cg==Cg==[Qq]
[q labels = “top”]Label the following.
| ph 6 | pH 8 |
| ____________ | ____________ |
[l]more acidic
[fx] No. Please try again.
[f*] Great!
[l]more basic
[fx] No, that’s not correct. Please try again.
[f*] Great!
[q labels = “top”]Compare the following:
| ph 3 | pH 5 |
| ____________ | ____________ |
[l]more H+
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]more OH–
[fx] No, that’s not correct. Please try again.
[f*] Great!
[q labels = “top”]Label the following.
| ph 3 | pH 9 |
| ____________ | ____________ |
[l]less basic
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]more basic
[fx] No, that’s not correct. Please try again.
[f*] Good!
[q]Compared to a solution that’s pH 3, a solution that’s pH 1 is ___ times more acid.
[c]dHdpY2U=[Qq]
[c]dGhyZWUgdGltZXM=[Qq]
[c]dGVuIHRpbWVz[Qq]
[c]MTAwIH RpbWVz[Qq]
[f]Tm8uIFRoZSBwSCBzY2FsZSB3b3JrcyBieSBwb3dlcnMgb2YgMTAgKG11Y2ggbGlrZSB0aGUgUmljaHRlciBzY2FsZSBmb3IgZWFydGhxdWFrZSBtYWduaXR1ZGUpLiBGb3IgZXhhbXBsZSwgYSBwSCBvZiA1IGlzIDEwIHRpbWVzIGZvciBhY2lkaWMgdGhhbiBhIHBIIG9mIDYuIFVzZSB0aGlzIHRvIGZpZ3VyZSBvdXQgdGhlIGRpZmZlcmVuY2UgaW4gYWNpZGl0eSBiZXR3ZWVuIHBIIDEgYW5kIHBoIDMu[Qq]
[f]Tm8uIFRoZSBwSCBzY2FsZSB3b3JrcyBieSBwb3dlcnMgb2YgMTAgKG11Y2ggbGlrZSB0aGUgUmljaHRlciBzY2FsZSBmb3IgZWFydGhxdWFrZSBtYWduaXR1ZGUpLiBGb3IgZXhhbXBsZSwgYSBwSCBvZiA1IGlzIDEwIHRpbWVzIGZvciBhY2lkaWMgdGhhbiBhIHBIIG9mIDYuIFVzZSB0aGlzIHRvIGZpZ3VyZSBvdXQgdGhlIGRpZmZlcmVuY2UgaW4gYWNpZGl0eSBiZXR3ZWVuIHBIIDEgYW5kIHBoIDMu[Qq]
[f]Tm8uIFRoZSBwSCBzY2FsZSB3b3JrcyBieSBwb3dlcnMgb2YgMTAgKG11Y2ggbGlrZSB0aGUgUmljaHRlciBzY2FsZSBmb3IgZWFydGhxdWFrZSBtYWduaXR1ZGUpLiBGb3IgZXhhbXBsZSwgYSBwSCBvZiA1IGlzIDEwIHRpbWVzIGZvciBhY2lkaWMgdGhhbiBhIHBIIG9mIDYuIFVzZSB0aGlzIHRvIGZpZ3VyZSBvdXQgdGhlIGRpZmZlcmVuY2UgaW4gYWNpZGl0eSBiZXR3ZWVuIHBIIDEgYW5kIHBoIDMu[Qq]
[f]WWVzLiBUaGUgcEggc2NhbGUgd29ya3MgYnkgcG93ZXJzIG9mIDEwIChtdWNoIGxpa2UgdGhlIFJpY2h0ZXIgc2NhbGUgZm9yIGVhcnRocXVha2UgbWFnbml0dWRlKSBBIHBIIG9mIDEgaXMgMTAwWCBtb3JlIGFjaWRpYyB0aGFuIGEgcEggb2YgMy4=
Cg==[Qq]
[q]Compared to a solution that’s pH 7, a solution that’s pH 8 is ___ times more basic.
[c]dHdpY2U=[Qq]
[c]dGhyZWUgdGltZXM=[Qq]
[c]dGVuIH RpbWVz[Qq]
[c]MTAwIHRpbWVz[Qq]
[f]Tm8uIFRoZSBwSCBzY2FsZSB3b3JrcyBieSBwb3dlcnMgb2YgMTAgKG11Y2ggbGlrZSB0aGUgUmljaHRlciBzY2FsZSBmb3IgZWFydGhxdWFrZSBtYWduaXR1ZGUpLiBVc2UgdGhhdCB0byBmaWd1cmUgb3V0IGhvdyBtdWNoIG1vcmUgYmFzaWMgOCBpcyB0aGFuIDcu[Qq]
[f]Tm8uIFRoZSBwSCBzY2FsZSB3b3JrcyBieSBwb3dlcnMgb2YgMTAgKG11Y2ggbGlrZSB0aGUgUmljaHRlciBzY2FsZSBmb3IgZWFydGhxdWFrZSBtYWduaXR1ZGUpLiBVc2UgdGhhdCB0byBmaWd1cmUgb3V0IGhvdyBtdWNoIG1vcmUgYmFzaWMgOCBpcyB0aGFuIDcu[Qq]
[f]WWVzLiBUaGUgcEggc2NhbGUgd29ya3MgYnkgcG93ZXJzIG9mIDEwLiBTbyBhIHNvbHV0aW9uIHRoYXQmIzgyMTc7cyBwSCA4IGlzIDEwIHRpbWVzIG1vcmUgYmFzaWMgdGhhbiBvbmVzIHRoYW4gb25lIHRoYXQmIzgyMTc7cyBwSCA3Lg==[Qq]
[f]Tm8uIFRoZSBwSCBzY2FsZSB3b3JrcyBieSBwb3dlcnMgb2YgMTAgKG11Y2ggbGlrZSB0aGUgUmljaHRlciBzY2FsZSBmb3IgZWFydGhxdWFrZSBtYWduaXR1ZGUpLiBVc2UgdGhhdCB0byBmaWd1cmUgb3V0IGhvdyBtdWNoIG1vcmUgYmFzaWMgOCBpcyB0aGFuIDcu
Cg==[Qq]
[q]A solution that is 1000 times less basic than a solution that is pH 12 would have a pH of ___
[textentry single_char=”true”]
[c]OQ ==[Qq]
[f]RXhjZWxsZW50LiBBIHNvbHV0aW9uIHRoYXQgaXMgMTAwMCB0aW1lcyBsZXNzIGJhc2ljIHRoYW4gYSBzb2x1dGlvbiB0aGF0IGlzIHBIIDEyIHdvdWxkIGhhdmUgYSBwSCBvZsKgOS4=[Qq]
[c]Kg==[Qq]
[f]Tm8uIEVhY2ggZGVjcmVhc2UgaW4gcEggbnVtYmVyIHJlcHJlc2VudHMgYSAxMCB0aW1lcyBkZWNyZWFzZSBpbiBhY2lkaXR5LiBTbywgcGggMTEgaXMgMTAgdGltZXMgbGVzcyBiYXNpYyB0aGFuIDEyLCBhbmQgcEggMTAgaXMgMTAwIHRpbWVzIGxlc3MgYmFzaWMgdGhhbiAxMi4gV2hhdCBwSCBpcyAxMDAwIHRpbWVzIGxlc3MgYmFzaWMgdGhhbiAxMj8=
Cg==[Qq]
[q]A solution that is 100 times less acidic than a solution that is pH 2 would have a pH of ___
[textentry single_char=”true”]
[c]NA ==[Qq]
[f]RXhjZWxsZW50LiBBIHNvbHV0aW9uIHRoYXQgaXMgMTAwIHRpbWVzIGxlc3MgYWNpZGljwqB0aGFuIGEgc29sdXRpb24gdGhhdCBpcyBwSCAyIHdvdWxkIGhhdmUgYSBwSCBvZiA0Lg==[Qq]
[c]Kg==[Qq]
[f]Tm8uIEVhY2ggaW5jcmVhc2XCoGluIHBIIG51bWJlciByZXByZXNlbnRzIGEgMTAgdGltZXMgZGVjcmVhc2UgaW4gYWNpZGl0eS7CoFNvLCBwaCAzwqBpcyAxMCB0aW1lcyBsZXNzIGFjaWRpY8KgdGhhbiBwSCAyLsKgV2hhdCBwSCBpcyAxMDAgdGltZXMgbGVzcyBhY2lkaWPCoHRoYW4gcEggMj8=[Qq]
[/qwiz]
5. Buffers
A buffer is a substance that when added to a solution, enables that solution to resist changes in pH when an acid or a base is added to it (note: this definition is a slightly modified version of one in the New Oxford American Dictionary). Blood, for example, resists changes in its pH much more than an equivalent quantity of water. That’s because blood contains buffers that are capable of soaking up hydrogen ions (H+) when acids enter the blood, and which release hydrogen ions when blood’s pH starts to rise.
As described in Campbell, Biology, 9th Edition, one of the buffers which enables blood to do this is carbonic acid. The formula for carbonic acid is H2CO3. Carbonic acid can dissociate by losing a hydrogen ion, making it into the bicarbonate ion (HCO3–), which is a base. You can see this in the chemical reaction below.
H2CO3 → HCO3– + H+
Carbonic acid itself is a weak acid. When added to water, a small amount of carbonic acid will dissociate and release H+ into the solution (which is why it’s an acid). But if another acid is added to the same solution, then the H+ released by the second acid will combine with the bicarbonate ion, creating more carbonic acid. Because carbonic acid is a buffer, that decreases the pH of the solution (making it less acidic) by absorbing the H+. By contrast, when a base is added to the solution (causing the pH to rise), more carbonic acid will dissociate, releasing H+ into the solution, causing the pH to fall.
6. Conclusion: Acids, Bases, pH, and Life
Acids and bases are incredibly important to understanding how living things work. One of the most important aspects of homeostasis in any organism is keeping the pH in a cell’s cytoplasm, outside the cell, and or within a body compartment (the stomach, or the inside of a blood vessel) within a very specific range. Exceeding that range, even by a little bit, can be harmful or fatal. We’ve seen above how buffers help maintain the pH of the blood. We’ll continue to see, throughout this course, the variety of adaptations that organisms have for controlling pH.
Links
This is the last tutorial in this series about the chemistry and properties of water. Use the menu above to choose your next topic.
