1. Life requires free energy
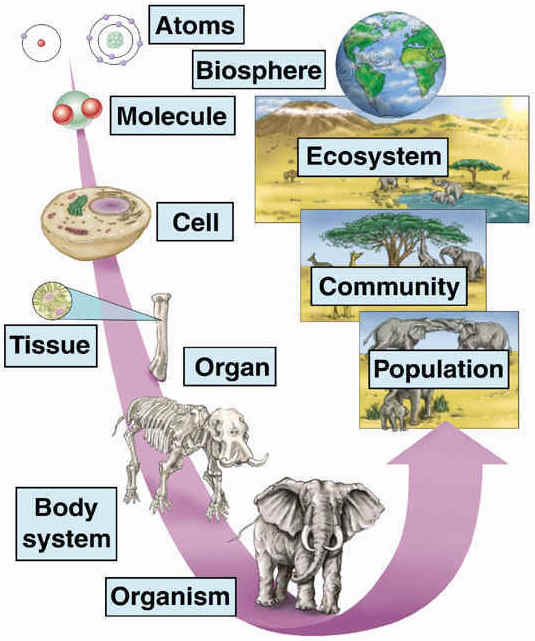 Living things — anything from an E. coli bacterium to a redwood tree to a human being like yourself — are complex and highly ordered systems. This complexity and order can be found in the molecules, organelles, cells, tissues, and organs that make up organisms, and it continues in higher levels of biological organization as well.
Living things — anything from an E. coli bacterium to a redwood tree to a human being like yourself — are complex and highly ordered systems. This complexity and order can be found in the molecules, organelles, cells, tissues, and organs that make up organisms, and it continues in higher levels of biological organization as well.
Complexity and order are inherently unstable conditions. That’s because of entropy, the universal tendency of any system to spontaneously become more disorganized.
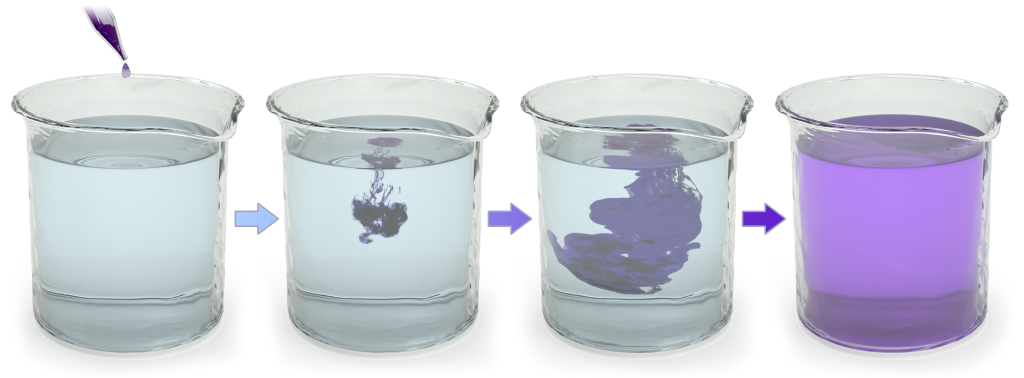
Add a drop of ink to a glass of water. Over time, the ink spreads out, becoming a diffuse mixture of ink and water. That’s entropy.
Entropy is why complex systems wear down over time. Think of any machine (a bicycle, an electrical motor, a seesaw, etc.). As you use it (unless you do the work of maintaining it and repairing it) it’ll eventually wear down. That’s entropy. Think of your bedroom. Unless you put energy into maintaining its order, your use of the room will cause it to become more disorganized over time. That’s entropy. And it’s true of any living organism Over time, organisms wear out, break down, and eventually die and decompose.
Entropy is a basic feature of the universe. It’s often called “time’s arrow:” you know that a video sequence is running backward when scenes progress from less organized to more organized, as in a movie scene where a broken glass of juice jumps from the floor up onto a table, puts itself back together and refills itself. But even though the universe is constantly moving from more organized to less organized, living things are temporarily capable of maintaining, growing, and reproducing highly organized structures. How?
Living things create, maintain, and reproduce life’s organization by harvesting and using what’s called free energy. Free energy (also known as Gibbs Free Energy) is energy that can be harvested to power work. The water flowing down a river can be harnessed to turn a turbine: that’s free energy. Sunlight can be harnessed to create electricity in a photovoltaic cell: that’s free energy. The chemical energy in gasoline can be harnessed to power an internal combustion engine and drive a car. That’s free energy.
Here are two forms of free energy that are used by living things:
- Sunlight to power photosynthesis.
- The chemical energy in carbohydrates or lipids to power cellular respiration.
To survive, an organism’s free energy input needs to exceed its overall energy use. If it doesn’t, the complex order that underlies life won’t be maintained. Entropy wins. Death results.
2. Living Things Fight Entropy through Coupling Exergonic Reactions to Endergonic Ones
The chemical reactions that occur in living things are of two types: exergonic and endergonic.
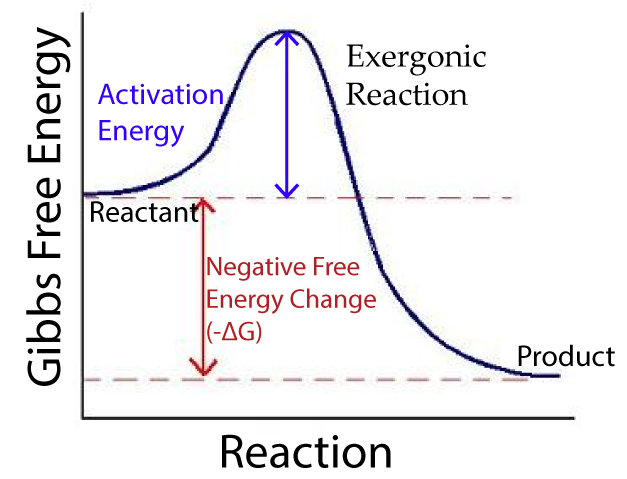 In an exergonic reaction, the reactants have more free energy than the products. As the reaction proceeds, free energy is released, and that released energy can be harnessed to perform work.
In an exergonic reaction, the reactants have more free energy than the products. As the reaction proceeds, free energy is released, and that released energy can be harnessed to perform work.
In the diagram on the right, the free energy change is represented by ΔG. In an exergonic reaction, the free energy change is negative and represented as -ΔG.
In general, exergonic reactions increase entropy and occur spontaneously. Spontaneous, in this context, doesn’t mean that exergonic reactions happen quickly or even on their own. It means that apart from the activation energy that’s needed to get them started, they don’t need any additional energy to run their course.
Cellular respiration is an exergonic reaction. Most hydrolysis reactions, in which polymers are broken down into monomers, are also exergonic.
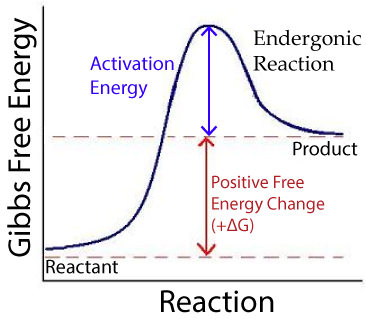
Endergonic reactions require energy. They’re not spontaneous, meaning that they require energy to run their course. In general, endergonic reactions decrease entropy.
Photosynthesis is an endergonic reaction. So are most dehydration synthesis reactions.
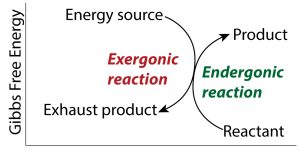
So how does life persist and thrive in the face of entropy? By coupling exergonic reactions to endergonic ones. The general scheme is shown on the right. The free energy released from an exergonic process is coupled with an endergonic process. For example, light is emitted from the sun in an exergonic reaction (nuclear fusion at the sun’s core). The source is hydrogen; the exhaust is helium. That conversion releases free energy in the form of sunlight. Sunlight drives the endergonic reactions of photosynthesis, which create carbohydrates (the product) from carbon dioxide and water (the reactants).
Now those carbohydrates (and the other biomolecules made from them) are themselves a source of free energy. Those molecules serve as an energy source to power cellular respiration (an exergonic reaction), resulting in carbon dioxide and water (the exhaust products). The free energy released is now available to power all of the endergonic reactions that occur in cells.
Usually, the coupling of exergonic processes to endergonic ones described above doesn’t happen directly but through intermediate molecules. Most commonly, that molecule is ATP, which will meet below. But first, let’s consolidate what we learned above.
3. Checking Understanding: Entropy and Energy Coupling
[qwiz qrecord_id=”sciencemusicvideosMeister1961-Entropy and Energy Coupling (2.0)”]
[h] Entropy and Energy Coupling
[q] The universal tendency of the universe to move from more organized to less organized is called [hangman].
[c]IGVudHJvcHk=[Qq]
[f]IEdvb2Qh[Qq]
[q] [hangman] energy is the type of energy that can be harvested to power work.
[c]IEZyZWU=[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[q] In the diagram below, graph 1 shows the course of a(n) [hangman] reaction. This type of reaction [hangman] free energy that can be used to perform [hangman].
[c]IGV4ZXJnb25pYw==[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IHJlbGVhc2Vz[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[c]IHdvcms=[Qq]
[f]IEdvb2Qh[Qq]
[q] In the diagram below, graph 2 shows the course of a(n) [hangman] reaction. In this type of reaction, entropy [hangman], and the products have [hangman] energy than the reactants.
[c]IGVuZGVyZ29uaWM=[Qq]
[f]IENvcnJlY3Qh[Qq]
[c]IGRlY3JlYXNlcw==[Qq]
[f]IEdyZWF0IQ==[Qq]
[c]IG1vcmU=[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[q] A reaction like photosynthesis is best represented by which of the graphs below?
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IFllcy4gUGhvdG9zeW50aGVzaXMgaXMgYW4gZW5kZXJnb25pYyByZWFjdGlvbiBhbmQgaXMgYmVzdCByZXByZXNlbnRlZCBieSBncmFwaCBudW1iZXIgMi4=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBJbiBwaG90b3N5bnRoZXNpcywgdGhlIHByb2R1Y3RzIGhhdmUgbW9yZSBlbmVyZ3kgdGhhbiB0aGUgcmVhY3RhbnRzLiBIb3cgd291bGQgdGhhdCBiZSByZXByZXNlbnRlZD8=[Qq]
[c]IEVudGVyIGxldHRlcg==[Qq]
[q] In both diagrams, activation energy is represented by which number or letter?
[textentry single_char=”true”]
[c]IG Q=[Qq]
[f]IFllcy4gVGhlIGxldHRlciAmIzgyMjA7ZCYjODIyMTsgcmVwcmVzZW50cyBhY3RpdmF0aW9uIGVuZXJneS4=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50IGZvciBuZXh0IHRpbWUuIFRoZSBhY3RpdmF0aW9uIGVuZXJneSBpcyB0aGUgJiM4MjIwO3B1c2gmIzgyMjE7IHJlcXVpcmVkIHRvIGdldCBhIHJlYWN0aW9uIHN0YXJ0ZWQu[Qq]
[c]IEVudGVyIGxldHRlcg==[Qq]
[q] Which reaction is not spontaneous and requires an input of energy to run its course?
[textentry single_char=”true”]
[c]ID I=[Qq]
[f]IFllcy4gTnVtYmVyIDIgcmVwcmVzZW50cyBhbiBlbmRlcmdvbmljIHJlYWN0aW9uLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50IGZvciBuZXh0IHRpbWUuIExvb2sgZm9yIHRoZSByZWFjdGlvbiB3aGVyZSB0aGUgcHJvZHVjdHMgaGF2ZSBtb3JlIGVuZXJneSB0aGFuIHRoZSByZWFjdGFudHMu[Qq]
[c]IEVudGVyIGxldHRlcg==[Qq]
[q] Cellular respiration is best represented by which of the graphs below?
[textentry single_char=”true”]
[c]ID E=[Qq]
[f]IFllcy4gQ2VsbHVsYXIgcmVzcGlyYXRpb24gaXMgYW4gZXhlcmdvbmljIHJlYWN0aW9uIGFuZCBpcyBiZXN0IHJlcHJlc2VudGVkIGJ5IGdyYXBoIDEu[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50IGZvciBuZXh0IHRpbWUuIExvb2sgZm9yIHRoZSByZWFjdGlvbiB3aGVyZSB0aGUgcHJvZHVjdHMgaGF2ZSBsZXNzIGVuZXJneSB0aGFuIHRoZSByZWFjdGFudHMu[Qq]
[c]IEVudGVyIGxldHRlcg==[Qq]
[q] Which letter represents an endergonic reaction?
[textentry single_char=”true”]
[c]IE I=[Qq]
[f]IFllcy4gJiM4MjIwO0ImIzgyMjE7IHJlcHJlc2VudHMgYW4gZW5kZXJnb25pYyByZWFjdGlvbi4=[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50IGZvciBuZXh0IHRpbWUuIExvb2sgZm9yIHRoZSByZWFjdGlvbiB3aGVyZSB0aGUgcHJvZHVjdHMgaGF2ZSBtb3JlIGVuZXJneSB0aGFuIHRoZSByZWFjdGFudHMu[Qq]
[c]IEVudGVyIGxldHRlcg==[Qq]
[q] Which number represents the source of the energy that makes reaction “B” possible?
[textentry single_char=”true”]
[c]ID E=[Qq]
[f]IFllcy4gJiM4MjIwOzEmIzgyMjE7IHJlcHJlc2VudHMgdGhlIGVuZXJneS1yaWNoIHJlYWN0YW50IHRoYXQmIzgyMTc7cyBtYWtpbmcgcmVhY3Rpb24gJiM4MjIwO0ImIzgyMjE7IHBvc3NpYmxlLg==[Qq]
[c]ICo=[Qq]
[f]IE5vLiBIZXJlJiM4MjE3O3MgYSBoaW50IGZvciBuZXh0IHRpbWUuIFJlYWN0aW9uICYjODIyMDtBJiM4MjIxOyBpcyBjb3VwbGVkIHdpdGggcmVhY3Rpb24gJiM4MjIwO0IuJiM4MjIxOyBXaGF0JiM4MjE3O3MgdGhlIGVuZXJneSBzb3VyY2UgZm9yIHJlYWN0aW9uICYjODIyMDtCLiYjODIyMTs=[Qq]
[c]IEVudGVyIGxldHRlcg==[Qq]
[q]The diagram below represents the idea of energy [hangman]. Reaction “A” is a(n) [hangman] reaction that’s powering the [hangman] reaction represented by “B.”
[c]Y291cGxpbmc=[Qq]
[c]ZXhlcmdvbmlj[Qq]
[c]ZW5kZXJnb25pYw==[Qq]
[/qwiz]
4. ATP is Life’s Key Energy Coupler (and is also important for other reasons)
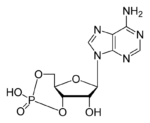
If there was a prize for the most important biological molecule, you might want to consider nominating ATP, which stands for adenosine triphosphate.
.jpg)
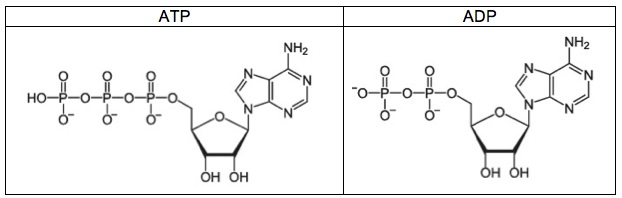
ATP is a nucleotide monomer. It’s composed of 3 subparts.
- Part 1 is the five-carbon sugar ribose.
- Part 2 is the nitrogenous base adenine, which is one of the letters of the genetic code.
- Part 3 consists of three phosphate groups (which is where the “triphosphate” part of ATP’s name comes from). These phosphate groups are used for energy transfer.
ATP is life’s energy carrier. It’s how the cells which make up every living thing store and release energy to perform the work of life. Movement, synthesis of polymers, cellular reproduction, and almost every other endergonic thing that cells do is powered by ATP.
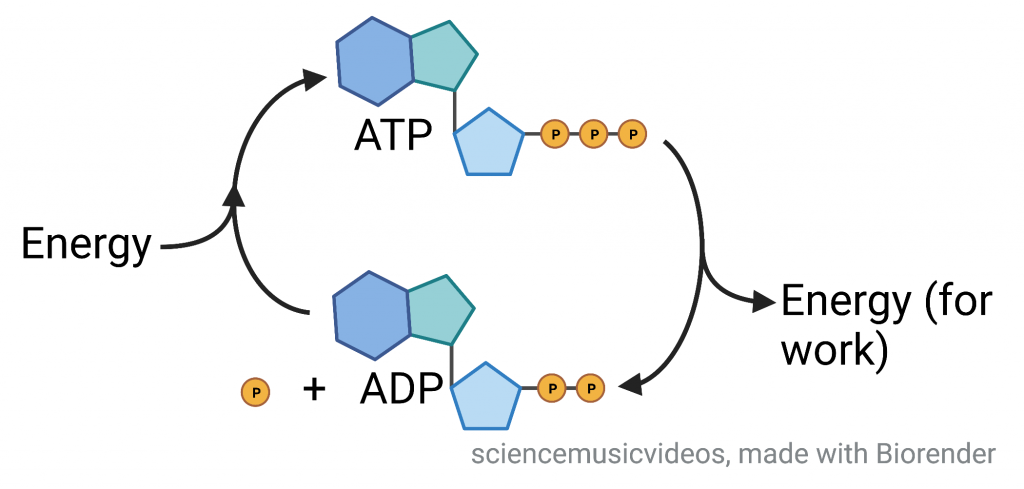
When cells need to perform work, they convert ATP into its lower energy counterpart, ADP and phosphate. ADP’s structure is identical to ATP’s except for the fact that ADP has two phosphate groups instead of three. Once released from ATP, the phosphate group is called “inorganic phosphate,” and represented by the symbol “Pi“)
Hydrolyzing the bond between the 2nd and 3rd phosphate groups is always accompanied by the formation of a bond between Pi and something else. Because the formation of that new bond releases more energy than is required to break the terminal phosphate off of ATP, the overall reaction is exergonic.
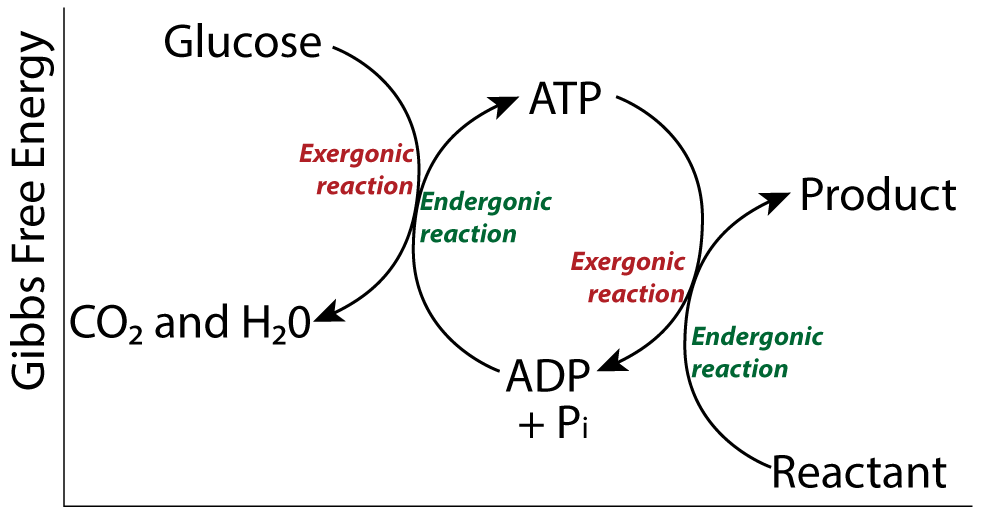 Every cell is independent in terms of ATP formation and consumption. To set themselves up to perform the work of fighting entropy, growing, and reproducing, cells need to continually replenish their supply of ATP. To do this, energy from an exergonic process (such as cellular respiration) is coupled to the formation of ATP from ADP and inorganic phosphate (an endergonic process). Then, the conversion of ATP to ADP and Pi (an exergonic process) can be coupled to any endergonic process required to sustain life.
Every cell is independent in terms of ATP formation and consumption. To set themselves up to perform the work of fighting entropy, growing, and reproducing, cells need to continually replenish their supply of ATP. To do this, energy from an exergonic process (such as cellular respiration) is coupled to the formation of ATP from ADP and inorganic phosphate (an endergonic process). Then, the conversion of ATP to ADP and Pi (an exergonic process) can be coupled to any endergonic process required to sustain life.
ATP is produced from ADP and Pi during both cellular respiration and photosynthesis. Understanding how that happens is a key part of AP Bio Unit 3.
Finally, if ATP’s central role in energy metabolism weren’t enough, ATP serves two other crucial roles in cells.
- ATP is information. It’s one of the four “letters” in RNA.
-
Cyclic AMP is an important signaling molecule. A modified form of ATP, cyclic AMP (adenosine monophosphate) is an important signaling molecule. When cells receive hormonal signals, ATP gets modified into a cyclic AMP, which carries the message deep within the cytoplasm, eliciting a cellular response.
5. Quiz: ATP is at the Center of Biology
[qwiz random=”false” style “width = 540px” qrecord_id=”sciencemusicvideosMeister1961-ATP Quiz (2.0)”]
[h]ATP is at the center of biology: Quiz
[i]
[q labels = “top”]Let’s try some labeling.
[l]adenine (nitrogenous base)
[fx] No. Please try again.
[f*] Great!
[l]three phosphate groups
[fx] No. Please try again.
[f*] Excellent!
[l]ribose sugar
[fx] No. Please try again.
[f*] Great!
[q]In a nucleotide polymer such as RNA, this part is one of the four letters.
[textentry single_char=”true”]
[c]Mg ==[Qq]
[f]WWVzLiAmIzgyMjA7MiYjODIyMTsgaXPCoGFkZW5pbmUsIG9uZSBvZiB0aGUgZm91ciBsZXR0ZXJzIG9mIHRoZSBjaGVtaWNhbCBhbHBoYWJldCB0aGF0IG1ha2VzIHVwIHRoZSBnZW5ldGljIGNvZGUu[Qq]
[c]Kg==[Qq]
[f]Tm8uIExvb2sgZm9yIHRoZSBuaXRyb2dlbm91cyBiYXNlLiBKdXN0IHRoaW5rIGFib3V0IHdoaWNoIHBhcnQgY291bGQgYmUgZGVzY3JpYmVkIGFzICYjODIyMDtuaXRyb2dlbm91cy4mIzgyMjE7
Cg==[Qq]
[q]Which part is the sugar ribose?
[textentry single_char=”true”]
[c]MQ ==[Qq]
[f]WWVzLiAmIzgyMjA7MSYjODIyMTsgaXPCoHRoZSBzdWdhciByaWJvc2Uu[Qq]
[c]Kg==[Qq]
[f]Tm8uIFN1Z2FycyB0eXBpY2FsbHkgaGF2ZSBhIGhleGFnb25hbCAoc2l4LXNpZGVkKSBvciBwZW50YWdvbmFsICg1IHNpZGVkKSBzdHJ1Y3R1cmUuIFdoaWNoIG9mIHRoZSBudW1iZXJlZCBwYXJ0cyBhYm92ZSBsb29rcyBsaWtlIGEgc3VnYXI/
Cg==[Qq]
[q]Which part is involved in energy transfer?
[textentry single_char=”true”]
[c]Mw ==[Qq]
[f]WWVzLiBUaGUgcGhvc3BoYXRlIGdyb3VwcyBhdCAmIzgyMjA7MyYjODIyMTsgYXJlIHdoZXJlIGFuZCBob3cgQVRQIHN0b3JlcyBhbmQgcmVsZWFzZXMgZW5lcmd5Lg==[Qq]
[c]Kg==[Qq]
[f]Tm8uIEl0JiM4MjE3O3MgdGhlIHBob3NwaGF0ZSBncm91cHMgdGhhdCBwbGF5IHRoaXMgcm9sZS4gV2hpY2ggaXMgdGhlIG9ubHkgcGFydCB0aGF0IGNvdWxkIGJlIGEgcGhvc3BoYXRlIGdyb3VwPw==
Cg==[Qq]
[q]ATP is a nucleotide [hangman]
[c]bW9ub21lcg==[Qq]
[f]WWVzLiBBVFAgaXMgYSBudWNsZW90aWRlIA==bW9ub21lcg==Lg==
Cg==[Qq]
[q]Which molecule below is ATP?
| 1 | 2 | 3 |
[textentry single_char=”true”]
[c]Mw ==[Qq]
[f]WWVzLiBUaGUgdGhyZWUgcGhvc3BoYXRlIGdyb3VwcyBhdCAmIzgyMjA7MyYjODIyMTsgbWFrZSB0aGlzIG1vbGVjdWxlICYjODIyMDtBVFAsJiM4MjIxOyBvciA=YWRlbm9zaW5lIHRyaXBob3NwaGF0ZS4=[Qq]
[c]Kg==[Qq]
[f]Tm8uIENvdW50IHRoZSBudW1iZXIgb2YgcGhvc3BoYXRlIGdyb3VwcyBhdHRhY2hlZCB0byBlYWNoIG1vbGVjdWxlLiBXaGF0IGRvZXMgdGhlICYjODIyMDtUJiM4MjIxOyBpbiAmIzgyMjA7QVRQJiM4MjIxOyBzdGFuZCBmb3I/
Cg==[Qq]
[q]ATP (in a slightly modified form) also serves as one of the “letters” in which of the following polymers?
[c]RE5B[Qq]
[c]Uk 5B[Qq]
[c]cHJvdGVpbg==[Qq]
[c]cG9seXNhY2NoYXJpZGVz[Qq]
[f]Tm8sIGJ1dCB5b3UmIzgyMTc7cmUgdmVyeSBjbG9zZS4gQVRQIGlzIGEgbW9ub21lciBvZiBvbmUgb2YgdGhlIG51Y2xlb3RpZGUgcG9seW1lcnMsIGJ1dCBub3QgRE5BICh0aGUgb25lIHlvdSYjODIxNztyZSBwb3NzaWJseSBtb3N0IGZhbWlsaWFyIHdpdGgpLiBXaGF0JiM4MjE3O3MgdGhlIA==b3RoZXI=IG51Y2xlb3RpZGUgcG9seW1lcj8=[Qq]
[f]Q29ycmVjdCEgQVRQIChpbiBhIHNsaWdodGx5IG1vZGlmaWVkIGZvcm0pIGFsc28gc2VydmVzIGFzIG9uZSBvZiB0aGUgbGV0dGVycyBvZiBSTkEu[Qq]
[f]Tm8uIFRoZSBsZXR0ZXJzIHRoYXQgbWFrZSB1cCBwcm90ZWluIGFyZSBhbWlubyBhY2lkcy4gSXQmIzgyMTc7cyBvbmUgb2YgdGhlIHR3byBpbmZvcm1hdGlvbmFsIG51Y2xlb3RpZGVzIG9uIHRoZSBsaXN0Lg==[Qq]
[f]Tm8uIFBvbHlzYWNjaGFyaWRlcyBhcmUgY2FyYm9oeWRyYXRlIHBvbHltZXJzLiBUaGV5JiM4MjE3O3JlIG1hZGUgb2YgbW9ub21lcnMsIGJ1dCB0aGUgJiM4MjIwO2xldHRlciYjODIyMTsgbm90aW9uIGRvZXNuJiM4MjE3O3QgYXBwbHkgdG8gdGhlbS4gTWFrZSBhbm90aGVyIGNob2ljZSB3aGVuIHlvdSBzZWUgdGhpcyBxdWVzdGlvbiBhZ2Fpbi4=[Qq]
[q labels = “top”]
[l]ADP
[fx] No. Please try again.
[f*] Correct!
[l]ATP
[fx] No. Please try again.
[f*] Excellent!
[l]Energy from food
[fx] No, that’s not correct. Please try again.
[f*] Great!
[l]Energy released for work
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]Phosphate group
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[q]The complexity and order found in living things
[c]cmVxdWlyZXMgZW5lcmd5IHRvIG1haW50YWlu[Qq]
[c]Z29lcyBhZ2FpbnN0IHRoZSBwcm9jZXNzIG9mIGVudHJvcHk=[Qq]
[c]aXMgaGlnaGx5IHVuc3RhYmxl[Qq]
[c]YWxsIG9mIHRo ZSBhYm92ZQ==[Qq]
[q labels = “top”]Over time, in any organism, energy _____________ needs to exceed energy _________. If not, the result is ________.
[l]death
[fx] No. Please try again.
[f*] Correct!
[l]input
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]loss
[fx] No. Please try again.
[f*] Excellent!
[q labels = “top”]To power the work of staying alive, cells convert _____________ into its lower energy form: _______ and inorganic ___________.
[l]ADP
[fx] No, that’s not correct. Please try again.
[f*] Correct!
[l]ATP
[fx] No, that’s not correct. Please try again.
[f*] Good!
[l]phosphate
[fx] No. Please try again.
[f*] Excellent!
[q topic= “ATP”]During cellular respiration, the chemical energy in food is transformed into
[c]IHRoZSBjaGVtaWNhbC BlbmVyZ3kgaW4gQVRQ[Qq]
[c]Z2x1Y29zZQ==[Qq]
[c]IGNhcmJvbiBkaW94aWRl[Qq]
[f]IFllcy4gTGl2aW5nIHRoaW5ncyBoYXZlIHRvIHRyYW5zZmVyIHRoZSBlbmVyZ3kgaW4gZm9vZHMgKHN1Y2ggYXMgZ2x1Y29zZSkgaW50byB0aGUgZW5lcmd5IGluIEFUUC4=[Qq]
[f]IE5vLiBQcm9kdWN0aW9uIG9mIGdsdWNvc2UgaXMgd2hhdCBoYXBwZW5zIGR1cmluZyBwaG90b3N5bnRoZXNpcyAoYSB0b3BpYyB3ZSYjODIxNztsbCBhZGRyZXNzIGxhdGVyIGluIEFQIEJpbyBVbml0IDMp[Qq]
[f]IE5vLiBDYXJib24gZGlveGlkZSBpcyBvbmUgb2YgdGhlIHdhc3RlIHByb2R1Y3RzIG9mIGNlbGx1bGFyIHJlc3BpcmF0aW9uLCB3aGljaCBpcyB0aGUgcHJvY2VzcyB0aGF0IGNlbGxzIHVzZSB0byBnZXQgZW5lcmd5IGZyb20gZm9vZC4gQnV0IGNhcmJvbiBkaW94aWRlIGlzIGEgd2FzdGUgcHJvZHVjdC4gTG9vayBmb3Igc29tZXRoaW5nIHRoYXQgY2VsbHMgY2FuIHVzZSB0byBwb3dlciB0aGVpciBsaWZlIHByb2Nlc3Nlcy4=
[q topic= “ATP”]In this diagram of ATP, the phosphate groups are shown at
[c]IDE=[Qq]
[c]IDI=[Qq]
[c]ID M=[Qq]
[f]IE5vLiBOdW1iZXIgMSBpcyB0aGUgc3VnYXIsIHJpYm9zZS4gVG8gZmluZCB0aGUgcGhvc3BoYXRlIGdyb3VwcywgbG9vayBmb3IgdGhlIGNoZW1pY2FsIHN5bWJvbCBmb3IgcGhvc3Bob3J1cywg4oCYUC7igJk=[Qq]
[f]IE5vLiBOdW1iZXIgMiBpcyB0aGUgbml0cm9nZW5vdXMgYmFzZSwgYWRlbmluZS4gVG8gZmluZCB0aGUgcGhvc3BoYXRlIGdyb3VwcywgbG9vayBmb3IgdGhlIGNoZW1pY2FsIHN5bWJvbCBmb3IgcGhvc3Bob3J1cywg4oCYUC7igJk=[Qq]
[f]IFllcy4gTnVtYmVyIDMgcmVmZXJzIHRvIHRoZSBwaG9zcGhhdGUgZ3JvdXBzLCB3aGVyZSBBVFAgc3RvcmVzIGl0cyBlbmVyZ3ku
[q topic= “ATP”]In this diagram of ATP, the nitrogenous base adenine is found at
[c]IDE=[Qq]
[c]ID I=[Qq]
[c]IDM=[Qq]
[f]IE5vLiBOdW1iZXIgMSBpcyB0aGUgc3VnYXIsIHJpYm9zZS4gVG8gZmluZCB0aGUgbml0cm9nZW5vdXMgYmFzZSBhZGVuaW5lLCBsb29rIGZvciB0aGUgbml0cm9nZW4tY29udGFpbmluZyByaW5ncy4g4oCYTuKAmSBpcyB0aGUgc3ltYm9sIGZvciBuaXRyb2dlbi4=[Qq]
[f]IFllcyEgTnVtYmVyIDIgaXMgdGhlIG5pdHJvZ2Vub3VzIGJhc2UsIGFkZW5pbmUu[Qq]
[f]IE5vLiBOdW1iZXIgMyByZWZlcnMgdG8gdGhlIHBob3NwaGF0ZSBncm91cHMsIHdoZXJlIEFUUCBzdG9yZXMgaXRzIGVuZXJneS4gVG8gZmluZCB0aGUgbml0cm9nZW5vdXMgYmFzZSBhZGVuaW5lLCBsb29rIGZvciB0aGUgbml0cm9nZW4tY29udGFpbmluZyByaW5ncy4g4oCYTuKAmSBpcyB0aGUgc3ltYm9sIGZvciBuaXRyb2dlbi4=
[q topic= “ATP”]In this diagram of ATP, the sugar ribose is found at
[c]ID E=[Qq]
[c]IDI=[Qq]
[c]IDM=[Qq]
[f]IFllcy4gTnVtYmVyIDEgaXMgdGhlIHN1Z2FyLCByaWJvc2UsIHdoaWNoIHlvdSBjYW4gaWRlbnRpZnkgYnkgaXRzIHBlbnRhZ29uYWwgc2hhcGUu[Qq]
[f]IE5vLiBOdW1iZXIgMiBpcyB0aGUgbml0cm9nZW5vdXMgYmFzZSwgYWRlbmluZS4gWW91IGNhbiBpZGVudGlmeSB0aGUgc3VnYXIgcmlib3NlIGJ5IGl0cyBwZW50YWdvbmFsIHNoYXBlLg==[Qq]
[f]IE5vLiBOdW1iZXIgMyByZWZlcnMgdG8gdGhlIHBob3NwaGF0ZSBncm91cHMsIHdoZXJlIEFUUCBzdG9yZXMgaXRzIGVuZXJneS4gWW91IGNhbiBpZGVudGlmeSB0aGUgc3VnYXIgcmlib3NlIGJ5IGl0cyBwZW50YWdvbmFsIHNoYXBlLg==
[q topic= “ATP”]In this diagram of ATP, the part that contains chemical bonds that are broken down for energy is
[c]IDE=[Qq]
[c]IDI=[Qq]
[c]ID M=[Qq]
[f]IE5vLiBOdW1iZXIgMSBpcyB0aGUgc3VnYXIsIHJpYm9zZS4gSW4gQVRQLCBpdOKAmXMgdGhlIGxhc3QgcGhvc3BoYXRlIGdyb3VwIHRoYXQgZ2V0cyBicm9rZW4gb2ZmIHRoZSBtb2xlY3VsZSB0byByZWxlYXNlIGVuZXJneSBmb3IgY2VsbHVsYXIgd29yay4gV2hpY2ggbnVtYmVyIGlzIHBvaW50aW5nIHRvIHRoZSBwaG9zcGhhdGUgZ3JvdXBzPw==[Qq]
[f]IE5vLiBOdW1iZXIgMiBpcyB0aGUgbml0cm9nZW5vdXMgYmFzZSwgYWRlbmluZS4gSW4gQVRQLCBpdOKAmXMgdGhlIGxhc3QgcGhvc3BoYXRlIGdyb3VwIHRoYXQgZ2V0cyBicm9rZW4gb2ZmIHRoZSBtb2xlY3VsZSB0byByZWxlYXNlIGVuZXJneSBmb3IgY2VsbHVsYXIgd29yay4gV2hpY2ggbnVtYmVyIGlzIHBvaW50aW5nIHRvIHRoZSBwaG9zcGhhdGUgZ3JvdXBzPw==[Qq]
[f]IFllcy4gTnVtYmVyIDMgcmVmZXJzIHRvIHRoZSBwaG9zcGhhdGUgZ3JvdXBzLCB3aGVyZSBBVFAgc3RvcmVzIGl0cyBlbmVyZ3kuIEJ5IGJyZWFraW5nIG9mZiB0aGUgbGFzdCBwaG9zcGhhdGUgZ3JvdXAsIGNlbGxzIHJlbGVhc2UgZW5lcmd5IGZvciBjZWxsdWxhciB3b3JrLg==
[q topic= “ATP”]When ATP is converted to ADP and Phosphate, which chemical bond is broken?
[c]IE E=[Qq]
[c]IEI=[Qq]
[c]IEM=[Qq]
[f]IFllcy4gQnJlYWtpbmcgdGhlIGJvbmQgYXQg4oCYQeKAmSBjb252ZXJ0cyBBVFAgdG8gQURQIGFuZCBwaG9zcGhhdGUsIHJlbGVhc2luZyBlbmVyZ3kgZm9yIGNlbGx1bGFyIHdvcmsu[Qq]
[f]IE5vLiBUbyBjb252ZXJ0IEFUUCB0byBBRFAgYW5kIHBob3NwaGF0ZSwgdGhlIG91dGVybW9zdCBwaG9zcGhhdGUgZ3JvdXAgaXMgYnJva2VuIG9mZi4gV2hpY2ggYm9uZCB3b3VsZCBoYXZlIHRvIGJyZWFrIHRvIHJlbGVhc2UgdGhlIG91dGVybW9zdCBwaG9zcGhhdGUgZ3JvdXA/[Qq]
[f]IE5vLiBUbyBjb252ZXJ0IEFUUCB0byBBRFAgYW5kIHBob3NwaGF0ZSwgdGhlIG91dGVybW9zdCBwaG9zcGhhdGUgZ3JvdXAgaXMgYnJva2VuIG9mZi4gV2hpY2ggYm9uZCB3b3VsZCBoYXZlIHRvIGJyZWFrIHRvIHJlbGVhc2UgdGhlIG91dGVybW9zdCBwaG9zcGhhdGUgZ3JvdXA/
[q topic= “ATP”]Which molecule shown below is ADP?
[c]IDE=[Qq]
[c]ID I=[Qq]
[f]IE5vLiBBRFAgaXMgc2hvcnQgZm9yIOKAmGFkZW5vc2luZSBkaS1waG9zcGhhdGUu4oCZIFRoZSDigJhkaeKAmSBzdGFuZHMgZm9yIHR3byBhbmQgcmVmZXJzIHRvIHRoZSB0d28gcGhvc3BoYXRlIGdyb3Vwcy4gV2hpY2ggb2YgdGhlc2UgdHdvIG1vbGVjdWxlcyBoYXMgVFdPIHBob3NwaGF0ZSBncm91cHM/[Qq]
[f]IFllcy4gTW9sZWN1bGUgMiBpcyBBRFAsIHdoaWNoIHlvdSBjYW4gaWRlbnRpZnkgYnkgaXRzIHR3byBwaG9zcGhhdGUgZ3JvdXBzLg==
[q topic= “ATP”]Which molecule below has more stored chemical energy?
[c]ID E=[Qq]
[c]IDI=[Qq]
[f]IFllcy4gQVRQLCB3aXRoIGl0cyB0aHJlZSBwaG9zcGhhdGUgZ3JvdXBzLCBoYXMgbW9yZSBzdG9yZWQgY2hlbWljYWwgZW5lcmd5IHRoYW4gQURQLg==[Qq]
[f]IE5vLiBJbiB0aGUgQVRQLUFEUCBzeXN0ZW0sIGhhdmluZyB0aHJlZSBwaG9zcGhhdGUgZ3JvdXBzIG1lYW5zIGhhdmluZyBtb3JlIGVuZXJneSB0aGFuIGhhdmluZyB0d28uIE5leHQgdGltZSwgY2hvb3NlIEFUUCwgd2hpY2ggaGFzIHRocmVlIHBob3NwaGF0ZSBncm91cHMu
[q topic= “ATP”]When a cell needs to release a small amount of energy, it converts
[c]IEFUUCB0byBBRFAgYW 5kIHBob3NwaGF0ZQ==[Qq]
[c]IEFEUCBhbmQgcGhvc3BoYXRlIHRvIEFUUA==[Qq]
[f]IFllcy4gQ29udmVyc2lvbiBvZiBBVFAgdG8gQURQIGFuZCBwaG9zcGhhdGUgcmVsZWFzZXMgZW5lcmd5IGZvciBjZWxsdWxhciB3b3JrLg==[Qq]
[f]IE5vLiBDb252ZXJ0aW5nIEFEUCBhbmQgcGhvc3BoYXRlIHRvIEFUUCBpcyBob3cgY2VsbHMgU1RPUkUgZW5lcmd5LiBIb3cgZG8gY2VsbHMgcmVsZWFzZSBlbmVyZ3k/
[q topic= “ATP”]When a cell needs to store a small amount of energy, it converts
[c]IEFUUCB0byBBRFAgYW5kIHBob3NwaGF0ZQ==[Qq]
[c]IEFEUCBhbmQgcGhvc3 BoYXRlIHRvIEFUUA==[Qq]
[f]IE5vLiBDb252ZXJzaW9uIG9mIEFUUCB0byBBRFAgYW5kIHBob3NwaGF0ZSByZWxlYXNlcyBlbmVyZ3ku[Qq]
[f]IFllcy4gQ29udmVydGluZyBBRFAgYW5kIFBob3NwaGF0ZSB0byBBVFAgaXMgaG93IGNlbGxzIHN0b3JlIGVuZXJneS4=
[q topic= “ATP”]In this diagram of the ATP-ADP cycle, which letter represents energy release?
[c]IEE=[Qq]
[c]IE I=
[f]IE5vLiBMZXR0ZXIgQSBzaG93cyBBRFAgYW5kIFBob3NwaGF0ZSBiZWluZyBjb252ZXJ0ZWQgaW50byBBVFAsIHdoaWNoIGlzIGhvdyBjZWxscyBzdG9yZSBlbmVyZ3kgZm9yIGNlbGx1bGFyIHdvcmsu[Qq]
[f]IFllcy4gTGV0dGVyIEIgc2hvd3MgdGhlIGNvbnZlcnNpb24gb2YgQVRQIHRvIEFEUCBhbmQgcGhvc3BoYXRlLCB3aGljaCBpcyBob3cgY2VsbHMgcmVsZWFzZSBlbmVyZ3kgZm9yIGNlbGx1bGFyIHdvcmsu
[q topic= “ATP”]Which of the following best describes the flow of energy in cells performing cellular respiration?
[c]IEZvb2QgdG8gQURQIHRvIGNlbGwgd29yaw==[Qq]
[c]IGZvb2QgZGlyZWN0bHkgdG8gY2VsbCB3b3Jr[Qq]
[c]IEFUUCB0byBmb29k[Qq]
[c]IGZvb2QgdG8gQVRQIH RvIGNlbGwgd29yaw==[Qq]
[f]IE5vLiBBRFAgaXNu4oCZdCB0aGUgbW9sZWN1bGUgdGhhdOKAmXMgYnJva2VuIGRvd24gZm9yIGNlbGwgd29yay4gRmluZCBhIHBhdGggd2hlcmUgQVRQIHByZWNlZGVzIGNlbGwgd29yay4=[Qq]
[f]IE5vLiBDZWxscyBoYXZlIHRvIGNvbnZlcnQgZm9vZCBlbmVyZ3kgdG8gQVRQIGJlZm9yZSB0aGV5IGNhbiBwZXJmb3JtIHdvcmsuIEZpbmQgYSBwYXRoIHdoZXJlIEFUUCBwcmVjZWRlcyBjZWxsIHdvcmsu[Qq]
[f]IE5vLiBJbiBjZWxsdWxhciByZXNwaXJhdGlvbiwgZm9vZCBpcyB1c2VkIHRvIG1ha2UgQVRQLiBGaW5kIGEgcGF0aCB3aGVyZSBBVFAgcHJlY2VkZXMgY2VsbCB3b3JrLg==[Qq]
[f]IFllcy4gRm9yIGNlbGxzIHRvIGhhdmUgdGhlIHR5cGUgb2YgZW5lcmd5IHRoZXkgbmVlZCB0byBwZXJmb3JtIHdvcmssIHRoZXkgbXVzdCBmaXJzdCBjb252ZXJ0IHRoZSBjaGVtaWNhbCBlbmVyZ3kgaW4gZm9vZCBpbnRvIHRoZSBjaGVtaWNhbCBlbmVyZ3kgaW4gQVRQLCB3aGljaCB0aGV5IGNhbiB0aGVuIHVzZSB0byBwZXJmb3JtIGNlbGx1bGFyIHdvcmsu
[x]
[restart]
[/qwiz]
What’s next?
- Proceed to Topic 3.6, part 1 (the next tutorial in AP Bio Unit 3)
