Introduction
Our last tutorial focused on how enzymes work, and how they’re affected by changes in their environment. Now we’ll look at 1) how enzyme activity can be inhibited, and 2) how cells regulate enzyme activity.
Enzyme Inhibition
We’ve seen previously how enzymes have a pH optimum: a pH at which the enzyme’s active site has the specific 3-D shape for binding with its substrate. If the pH drops below or rises above that optimum, then the secondary, tertiary, and quaternary bonds that stabilize the enzyme’s shape can change. This, in turn, can change the enzyme’s shape. If the shape of the active site changes, then the enzyme’s ability to bind with its substrate will be diminished, making the enzyme less effective.
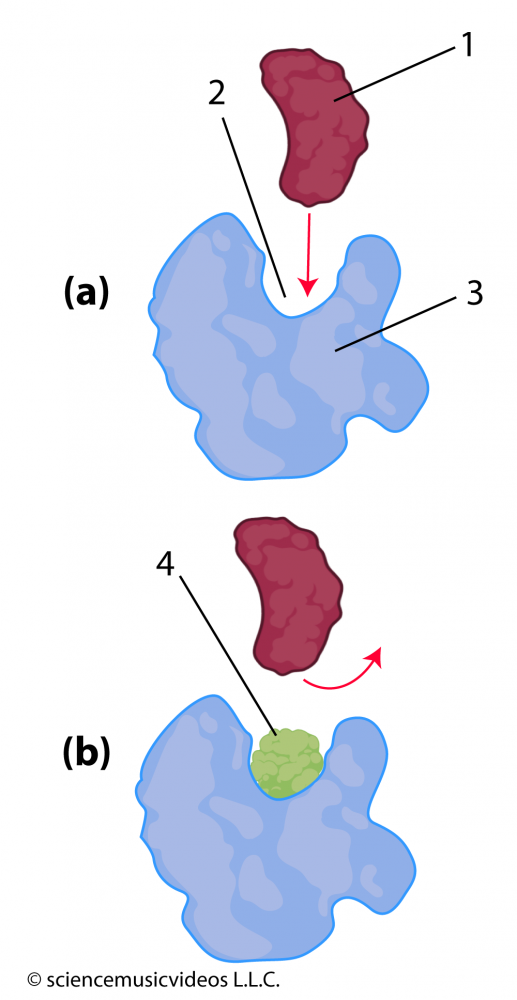 Molecules that enter an enzyme’s environment can also interfere with an enzyme’s ability to bind with its substrate. In diagram (a) at the left, you can see how an enzyme (“3”) has an active site (“2”) that fits with its substrate (“1”). However, if, as in diagram “b”, molecule “4” is also in the environment, then that molecule can also bind with the active site. This binding keeps the substrate from entering into the active site, blocking the conversion of substrate to product. That makes molecule “4” an inhibitor.
Molecules that enter an enzyme’s environment can also interfere with an enzyme’s ability to bind with its substrate. In diagram (a) at the left, you can see how an enzyme (“3”) has an active site (“2”) that fits with its substrate (“1”). However, if, as in diagram “b”, molecule “4” is also in the environment, then that molecule can also bind with the active site. This binding keeps the substrate from entering into the active site, blocking the conversion of substrate to product. That makes molecule “4” an inhibitor.
Because the inhibitor is competing with the substrate for access to the active site, this kind of inhibition is called competitive inhibition. The antibiotic penicillin, for example, is a competitive inhibitor of an enzyme that an entire class of bacteria uses to construct their cell walls (NCBI).
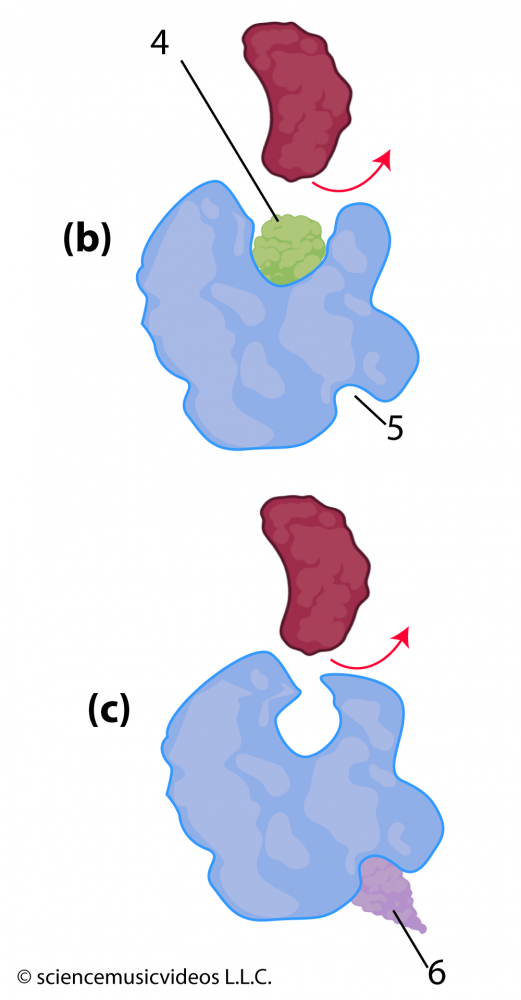 Inhibition can also be non-competitive. Notice that in the image of the enzyme at the right, there’s a deep cleft (at “5”) opposite the active site. Unlike the active site, which is a specific shape that evolved through natural selection to bind with the enzyme’s substrate, this cleft might not be a functional part of the enzyme. However, other molecules, such as “6” can snuggle into it. When they do, their interactions with the amino acids on that side of the enzyme can cause changes in secondary, tertiary, and quaternary bonds throughout the rest of the enzyme. If these changes alter the shape of the active site, then the enzyme’s substrate might not be able to bind with the active site, shutting down the enzyme’s activity.
Inhibition can also be non-competitive. Notice that in the image of the enzyme at the right, there’s a deep cleft (at “5”) opposite the active site. Unlike the active site, which is a specific shape that evolved through natural selection to bind with the enzyme’s substrate, this cleft might not be a functional part of the enzyme. However, other molecules, such as “6” can snuggle into it. When they do, their interactions with the amino acids on that side of the enzyme can cause changes in secondary, tertiary, and quaternary bonds throughout the rest of the enzyme. If these changes alter the shape of the active site, then the enzyme’s substrate might not be able to bind with the active site, shutting down the enzyme’s activity.
The poison cyanide is a competitive inhibitor of the enzymes used in cellular respiration. You can read more about other enzyme inhibitors and their uses in this Wikipedia article.
Just to make sure you’ve got it, take this quiz about competitive and non-competitive inhibition.
Enzyme Inhibition Quiz
[qwiz random=”true” use_dataset=”Enzyme Inhibition-SMV (AP)” dataset_intro=”false” qrecord_id=”sciencemusicvideosMeister1961-Enzyme Inhibition (M9)”] [h]
Enzyme Inhibition
[i]
[x][restart]
[/qwiz]
Enzyme Regulation
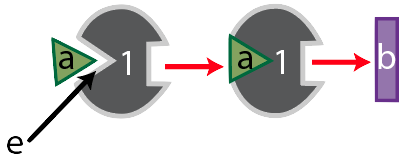
Meet enzyme “1.”
Its substrate is molecule “a,” which enzyme “1” binds with at its active site, shown at “e.” Together, the two form an enzyme-substrate complex, and when enzyme “1’s” work is done, it has converted substrate “a” into substance “b.”
Like many enzymes, enzyme “1” is part of a metabolic pathway. That’s because enzymes are like the workers (and increasingly, the robots) on an assembly line. These workers (or robots) do one thing. So if a cell needs to take a molecule and transform it into something quite different, it usually does so in several small steps, each of which is controlled by a different enzyme. Enzyme “1,” for example, is the first enzyme in a three-enzyme pathway, as shown below.
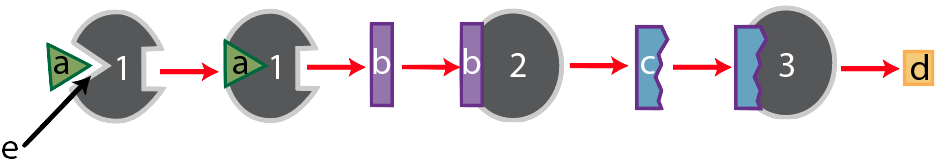
After enzyme “1” converts substrate “a” into substance “b,” substance “b” becomes the substrate for enzyme 2, which converts “b” into “c.” Finally, substance “c” becomes the substrate for enzyme “3,” which converts substrate “c” into the final product, “d.”
Now, let’s think of this in an evolutionary context. In the struggle for life, any organism that can more efficiently use resources will have an advantage. For example, looking at the pathway above, if the cells of an organism have enough of product “d,” then it would be advantageous to stop consuming substrate “a,” and to save it for later until product “d” is needed. But that would require turning enzyme “1” off. How can a cell turn an enzyme off?
One method is called feedback inhibition. The product, “d” has to somehow interact with enzyme “1” in a way that inactivates it. Here’s how this could work.
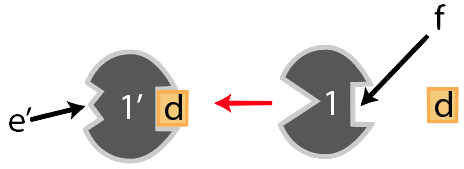
Notice that opposite enzyme “1’s” active site there’s a square-shaped indentation. It doesn’t catalyze any reactions, so it’s not itself an active site. But it does have a shape that complements substance “d.” And when “d” binds with “f,” interactions between “d” and the amino acids on that side of enzyme “1” cause changes throughout the enzyme in a way that changes the shape of enzyme 1’s. The result is that the active site can no longer bind with the original substrate. In other words, the product, when it binds with site “f,” turns the enzyme off. It feeds back to the first enzyme: hence, feedback inhibition.
Binding sites like “f” are called allosteric sites. Their most important property is that when something binds with them, it changes the enzyme’s active site. And in this case, when a molecule binds with an allosteric site in such a way that it makes the enzyme unable to bind with its substrate, then the process is called allosteric inhibition.
Here’s the entire pathway.
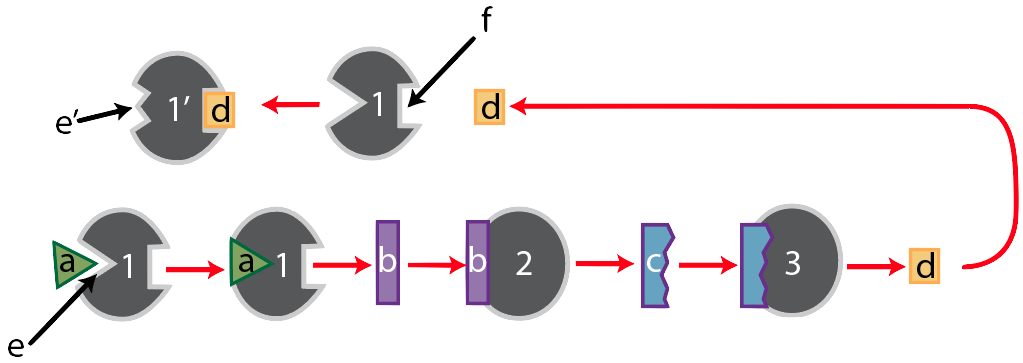
We’ve already walked through the bottom portion of the diagram, so let’s focus on the part that starts after the product of this pathway, substance “d,” has been produced. When there’s a lot of “d” around, then “d” will bind with “f,” the allosteric binding site of enzyme “1.” When it does, enzyme “1’s” active site will change its shape so that it no longer complements substrate “a,” which will shut down the entire pathway. Note that this only makes sense if you think of populations of enzymes and substrates. If there’s a lot of “d” produced, then the chance of enzyme “1” being in its active form is very low. Conversely, if there’s very little of “d,” then enzyme 1’s active site will be ready to complement substrate “a,” starting up that metabolic pathway.
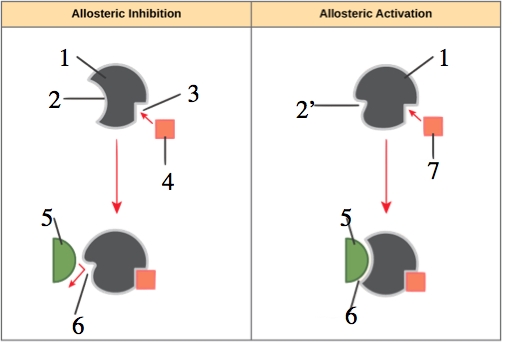
In the diagram to your left, you can see that allosteric regulation can involve inhibition or activation. The left side of the diagram shows inhibition, with an inhibitor (“4”) binding with an allosteric site (“3”) in such a way that the enzyme’s active site (“2”) changes shape (“6”) in such a way that it can no longer bind with the enzyme’s substrate (“5”).
The right side, by contrast, shows allosteric activation. In this case, the enzyme’s active site (shown at 2′) is unable to bind with the substrate (again “5”) until the allosteric activator binds with the enzyme’s allosteric site, changing the conformation of the active site (“6”) so that it can bind with the substrate.
Allosteric inhibition looks a lot like non-competitive inhibition. What’s the difference? Allosteric inhibition is a regulatory adaptation, used by cells to control enzyme catalyzed reactions within cells. Non-competitive is something that happens to cells when a foreign molecule is introduced into the cell’s (or organism’s) environment. The mechanism is the same. It’s the context that differs.
Enzyme Regulation Quiz
Got it? Take this quiz to see if you’ve mastered these concepts.
[qwiz random=”true” use_dataset=”SMV_Enzyme Regulation (AP)” dataset_intro=”false” qrecord_id=”sciencemusicvideosMeister1961-Enzyme Regulation (M9)”] [h]
Enzyme Regulation
[i]
[x][restart]
[/qwiz]
Next steps
- Proceed to Cellular Respiration the next module in Mr. W’s biology curriculum
- Return to the Energy and Enzymes Menu
