Introduction
Have you ever cleaned your bathroom at home? You probably used a base. Bleach is a very strong base. On the other hand, toilet bowl cleaners contain hydrochloric acid, which is also used in swimming pools to prevent algae build up on the floor and walls. Acids and bases are common in everyday life and you are probably more familiar with them than you think. Today we start looking at the chemistry of acids and bases.
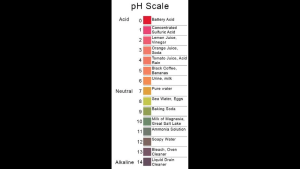
pH scale
pH is a measure of how acidic or basic a solution is. Recall that a solution is a liquid – the “solvent” – plus a substance- the “solute” – mixed or dissolved in the solvent. For the most part we will be talking about water solutions – water is the solvent – but other solutions can also be acidic or basic. The pH scale has a range from zero to 14. Zero is highly acidic; 14 is highly basic; 7 is neutral – neither basic nor acidic.
Another name for a basic solution is “alkaline.” Thus pH can also be said to measure acidity or alkalinity.
pH actually has a precise meaning. pH measures the relative amount of free hydrogen (H+) and hydroxyl (OH‾) ions in the solution. A solution that has more free hydrogen ions is acidic, whereas a solution that has more free hydroxyl ions is basic. These ions are reactive – they will combine with or split apart other substances. That is why cleaning products are often either highly acidic or highly basic – they’ll break down dirt, germs and other things you want to clean up.
Pure water is neutral. There are small amounts of free hydrogen ions and free hydroxyl ions in pure water – that is, some water molecules have dissociated – but as a result the number of hydrogen ions exactly matches the number of hydroxyl ions.
Non-neutral pH is caused by chemicals in the solute. Thus, pH is an important indicator of water that is changing chemically. Note, however, that there are some chemicals that don’t change the pH of water; salt is one example.
pH is reported in “logarithmic units”. Each unit change in pH (say from 4 to 3) represents a 10-fold change in the acidity or alkalinity of the solution. An acid having a pH of 3 is ten times more acidic than an acid having a pH of 4 and 100 times more acidic than an acid having a pH of 5.
[qwiz qrecord_id=”[email protected] are acids and bases”]
[q] If it stings when it gets into your eyes it is a(n) [hangman].
[c]IGFjaWQ=[Qq]
[f]IEV4Y2VsbGVudCE=[Qq]
[q multiple_choice=”true”] If the ph of a solution <7 it’s
[c]wqAgYW4g YWNpZA==[Qq]
[f]IGZhbnRhc3RpYy4gaWYgdGhlIHBIIG9mIGEgc29sdXRpb27CoCAmbHQ7IDcgaXQgaXMgYW4gYWNpZA==[Qq]
[c]IGJhc2U=[Qq]
[f]IE5vLiBhIGJhc2UgaGFzIGEgcEggJmd0OyA3[Qq]
[c]IG5ldXRyYWw=[Qq]
[f]IE5vLiBBIG5hdHVyYWwgc29sdXRpb24gaGFzIGEgcEggPTc=[Qq]
[q] The pH of distilled water is
[textentry single_char=”true”]
[c]ID c=
[f]IEZhbnRhc3RpYy7CoCBUaGUgcGggb2YgZGlzdGlsbGVkIHdhdGVyIGlzIDc=[Qq]
[c]ICo=[Qq]
[f]IEhpbnQhwqAgQWNpZHMgaGF2ZSBwSCAmbHQ7IDcgYW5kIGJhc2VzIGFyZSAmZ3Q7Nw==[Qq]
[/qwiz]
The first scientist of note to define acids and bases was a Swedish scientist named Svante Arrhenius (1859-1927).
The most common and simplest definition of pH is based on a substance’s behavior when water is the solvent. But this definition does not work for non-water solutions, so there is another definition that is a little more complicated, but is more general – the second definition works in ALL cases. The two definitions are named after the chemists who first came up with these definitions – “Arrhenius acids and bases” and “Brønsted-Lowry acids and bases.”
As we said, Arrhenius acids and bases are defined by a substance’s behavior in water. An Arrhenius acid contains hydrogen ions and those hydrogen ions, when combined with water molecules, will form hydronium ions- a water molecule with an extra hydrogen: H3O+. These hydronium ions are the key component of an Arrhenius acid.
On the other hand, an Arrhenius base exists when a substance is dissolved in water and creates a hydroxide ion (OH–) in the product. For example, bleach is sodium hypochlorite, NaClO, dissolved in water, which creates HOCl+ ions and NaOH– ions.
As we said an Arrhenius acid can best be defined as a substance which when dissolved in water, donates a hydrogen ion to a water molecule to make a hydronium ion, . By donating H+ ions, the acid molecules cause an increase in the hydronium ion concentration. The Arrhenius pH is based on the concentration of hydronium ions.
The dissociation of hydrochloric acid, HCl in water demonstrates the formation of hydronium:
HCl + H20 → H30+ + Cl–
An Arrhenius base, conversely, is a substance that when dissociated in water, will form a hydroxide ion,
The dissociation of sodium hydroxide is shown here:
The limitation of Arrhenius acids and bases is that water needs to be present in order for a substance to qualify. While water is present in many situations, it is not always the case, and acids often exist even in situations where water is not present. An acidic compound being dissolved in something such as toluene, , would not create the hydronium ions that meet the definition of an Arrhenius acid.
Similarly, the definition of Arrhenius bases runs into a small problem in some circumstances as well. A compound such as NaNH2 when dissolved in a liquid form of ammonia, , would not produce hydroxide ions.
Many compounds are thought to be either basic or acidic although they don’t meet the Arrhenius definition of acids and bases. However, the Arrhenius definitions are a great starting point for understanding the concept of acids and bases.
The Brønsted-Lowry Theory of Acids and Bases

In 1923, Danish chemist Johannes Brønsted and English chemist Thomas Lowry independently proposed new definitions for acids and bases. These definitions focus on proton transfer. A Brønsted-Lowry acid is any chemical that can donate a proton (H+) to another molecule. A Brønsted-Lowry base is any chemical that can accept a proton from another molecule. In short, a Brønsted-Lowry acid is a proton donor, while a Brønsted-Lowry base is a proton acceptor .
A Brønsted-Lowry acid is a proton donor, while a Brønsted-Lowry base is a proton acceptor.
Let us use the reaction of ammonia in water to demonstrate the Brønsted-Lowry definitions of an acid and a base. Ammonia and water molecules are reactants, while the ammonium ion and the hydroxide ion are products:
NH3 + H2O -> NH4+ + OH-
NH3(aq)+H2O(ℓ)↽−−⇀NH+4(aq)+OH−(aq)(1.12.1)(1.12.1)NH3(aq)+H2O(ℓ)↽−−⇀NH4+(aq)+OH−(aq)
What has happened in this reaction is that the water molecule has donated a hydrogen ion to the ammonia molecule, which then accepts the hydrogen ion. Look at the reaction below:
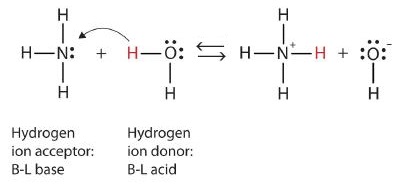
Since the water molecule donates a hydrogen ion to the ammonia, it is the Brønsted-Lowry acid, while the ammonia molecule— the hydrogen ion acceptor—is the Brønsted-Lowry base. Thus, ammonia is a base in both models.
Would an Arrhenius acid like hydrochloric acid still act like an acid in the Brønsted-Lowry sense? Yes, but we must understand what happens when HCl and water react. First, the hydrogen atom is a single proton surrounded by a single electron. To make the hydrogen ion, we remove the electron, leaving a bare proton. Would bare protons float around in aqueous solution? That answer would be NO. What happens is that the H+ ion attaches itself to H2O to make H3O+, which is called the hydronium ion. For most purposes, H+ and H3O+ represent the same species, but writing H3O+ instead of H+ shows that we see that there are no bare protons floating around in solution. Rather, these protons are actually attached to solvent molecules.
The Hydronium Ion
A proton in aqueous solution may be surrounded by more than one water molecule, leading to formulas like H5O+2H5O2+ or H9O+4H9O4+ rather than H3O+H3O+. It is simpler, however, to use H3O+H3O+ to represent the hydronium ion.
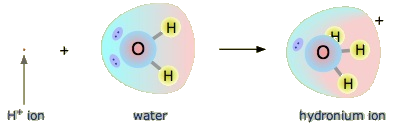
With this in mind, how do we define HCl as an acid in the Brønsted-Lowry sense? Consider what happens when HCl is dissolved in H2O:
HCl + H2O → H3O+ + Cl–
[qwiz qrecord_id=”[email protected] Formula”][q] What is the formula for the Hydronium Ion [hangman]
[c]IEgzTw==Kw==[Qq]
[/qwiz]
Bronstead Lowry Hypothesis
[qdeck qrecord_id=”[email protected] Flashcards”]
[q] According to the Bronsted Lowry Law what is an acid?
[a] an acid is a proton donor.
[q] According to the Bronsted Lowry Law what is a base?
[a] a base is a proton acceptor.
[/qdeck]
We can depict this process using Lewis electron dot diagrams:
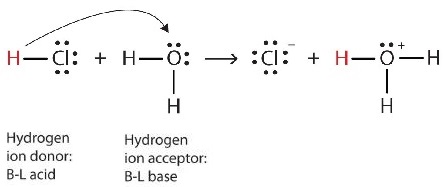
Now we see that a hydrogen ion is transferred from the HCl molecule to the H2O molecule to make chloride ions and hydronium ions. As the hydrogen ion donor, HCl acts as a Brønsted-Lowry acid; as a hydrogen ion acceptor, H2O is a Brønsted-Lowry base. So HCl is an acid not just in the Arrhenius sense but also in the Brønsted-Lowry sense. Moreover, by the Brønsted-Lowry definitions, H2O is a base in the formation of aqueous HCl. So the Brønsted-Lowry definitions of an acid and a base classify the dissolving of HCl in water as a reaction between an acid and a base—although the Arrhenius definition would not have labeled H2O a base in this circumstance.
- A Brønsted-Lowry acid is a proton (hydrogen ion) donor.
- A Brønsted-Lowry base is a proton (hydrogen ion) acceptor.
- All Arrhenius acids and bases are Brønsted-Lowry acids and bases as well. However, not all Brønsted-Lowry acids and bases are Arrhenius acids and bases.
Defining this in molecular terms, an acid is a molecule which will donate a hydrogen ion to the solution. A strong acid is an acid which will dissociate completely and it will result in an anion and a hydrogen ion.
On the other hand, a base is a molecule which can accept hydrogen (H+) ions from the solution. A strong base will dissociate completely and it will result in a cation and a hydroxide (OH–) ion.
Conjugate Acid-Base Pairs—
A conjugate acid is what is left of the acid after the proton is donated.
Likewise, a conjugate acid is formed by adding a proton to the base.
Consider HCl(aq) + H2O(l) H3O+(aq) + Cl–(aq).
- HCl and Cl– differ only in the whether the proton has been donated or accepted
- They are called a conjugate acid-base pair.
- Cl– is called the conjugate base.
- After HCl an (acid) loses its proton, it is converted into Cl– (a base).
- Therefore, HCl and Cl– are a conjugate acid-base pair.
- After H2O (a base) gains a proton, it is converted into H3O+ (an acid).
- H3O+ is the conjugate acid.
- Therefore, H2O and H3O+ are a conjugate acid-base pair.
Give the conjugate acid or conjugate base for each chemical below:
[qwiz qrecord_id=”[email protected] Acids/Bases”][q] What is the conjugate acid of NH3 [hangman]
[c]IE5INCs=[Qq]
[q] What is the conjugate base of HNO2 [hangman]
[c]IE5PMi0=
Cg==wqA=[Qq][q] What are the conjugate base pairs for the reaction HCO2H+H2O↔ HCO2– +H3O+
[hangman]
[c]IEhDTzJILUhDTzIt[Qq]
[/qwiz]
LEWIS Acid and Bases
Lewis, the infamous scientist of Lewis structures gave a new definition of acids and bases that emphasizes the shared electron pair (certainly he would!).
He said a Lewis acid is an electron pair acceptor and he said a Lewis base is an electron pair donor.
By this definition Lewis acids and bases do not need to contain protons so the Lewis definition is the most general definition of acids and bases.
But what are Lewis acids?
Usually they will have an incomplete octet.
They have the following characteristics
- They are usually Transition-metal ions.
- They have to have a a vacant orbital so an electron pair can be donated.
- Any Compound with multiple bonds can be a Lewis acids.
Look at this reaction
H2O(l) + CO2(g) H2CO3(aq)
Water is the electron pair donor and carbon dioxide as the electron pair acceptor.
Overall, the water (which we call a Lewis base) has donated a pair of electrons to the CO2 (which we call a Lewis acid).
Another way to view this is that Metal ions are positively charged and attract water molecules (the oxygen has a lone pair).
Also remember
The higher the charge, the higher thr pH.
The smaller the metal ion, the lower the Ph.
- Thus, the pH increases as the size of the ion increases (size increases from right to left and as you go down the Periodic table) and as the charge increases ( Na+ vs. Ca2+ and Zn2+ vs. Al3+).
NAMING ACIDS AND BASES
All acids contain hydrogen, so the name of an acid is based on the anion that goes with it.
A binary acid is an acid that consists of hydrogen and one other element. The most common binary acids contain a halogen. The acid name begins with the prefix hydro-. followed by the base name of the anion, followed by the suffix -ic.
For example….HCl=”Hydro” + “Chlorine” (Drop the -ine) so it is Chloric or Hydrochloric Acid
HI=”Hydro” + Iodine (Drop the -ine) so it is Iodic or Hydroiodic Acid
Naming Oxyacids
An oxyacid is an acid that consists of hydrogen, oxygen, and a third element. The third element is usually a nonmetal.
a. Oxyanions that end with –ite.
The name of the acid is the root of the anion followed by the
suffix -ous. There is no prefix.
b. Oxyanions that end with –ate.
The name of the acid is the root of the anion followed by the suffix -ic. There is no prefix.
Note
The base name for sulfur containing oxyacid is sulfur- instead of just sulf-. The same is true for a phosphorus containing oxyacid. The base name is phosphor- instead of simply phosph-.
H2S—–Hydrosulfuric Acid
NAMING BASES
There is no special system for naming bases. Since they all contain the OH− anion, names of bases end in hydroxide. The cation is simply named first. Some examples of names and formulas for bases are shown in the table below.
| Formula | Name |
| NaOH | sodium hydroxide |
| Ca(OH)2 | calcium hydroxide |
| NH4OH | ammonium hydroxide |
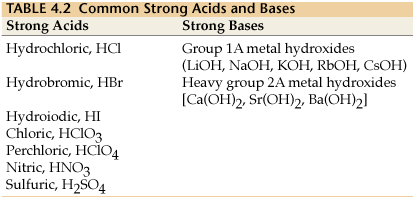
STRONG ACIDS AND BASES
Explanation:
Strong acids and strong bases are those species which dissociate/ionize essentially to completion upon being placed in aqueous solution.
Dissociation of a strong acid (hydrochloric for example):
HCl(g)→H+(aq)+Cl−(aq)
Dissociation of a strong base (sodium hydroxide for example):
NaOH(s)→Na+(aq)+OH−(aq)
Substances that ionize completely (which include strong acids and bases) are called strong electrolytes, and further include basically all water-soluble ionic compounds.
Strong acids and strong bases dissociate/ionize to completion upon being placed in aqueous solution.
Dissociation of hydrochloric acid:
HCl(g)→H+(aq)+Cl−(aq)
Dissociation of sodium hydroxide:
NaOH(s)→Na+(aq)+OH−(aq)
Substances that ionize completely (which include strong acids and bases) are called strong electrolytes, and include basically all water-soluble ionic compounds.
For a list of the strong acids and bases, try this: